Abstract
A soluble polysaccharide antigen from the cell wall of Rothia dentocariosa ATCC 17931 has been isolated, purified and characterized by serological and chemical procedures. The polysaccharide (RPS) was found to be located at the surface of cells grown under diverse environmental conditions, and could be easily detected on cells in pure culture or in clinical samples from humans and experimentally infected hamsters by fluorescent-antibody techniques. Fructose, glucose, galactose, and ribose were the major constituents of RPS. Although purified RPS was not immunogenic in rabbits, it was presumed to be a major antigen of the cell because it could specifically absorb approximately one-third of the antibody nitrogen in antisera prepared against whole cells of R. dentocariosa. Haptene inhibition studies indicated that fructose was the principal determinant of serological specificity in RPS. This polysaccharide was found to be serologically unique and did not cross-react with the polysaccharides and surface polymers of other oral actinomycetes and filamentous organisms.
Full text
PDF

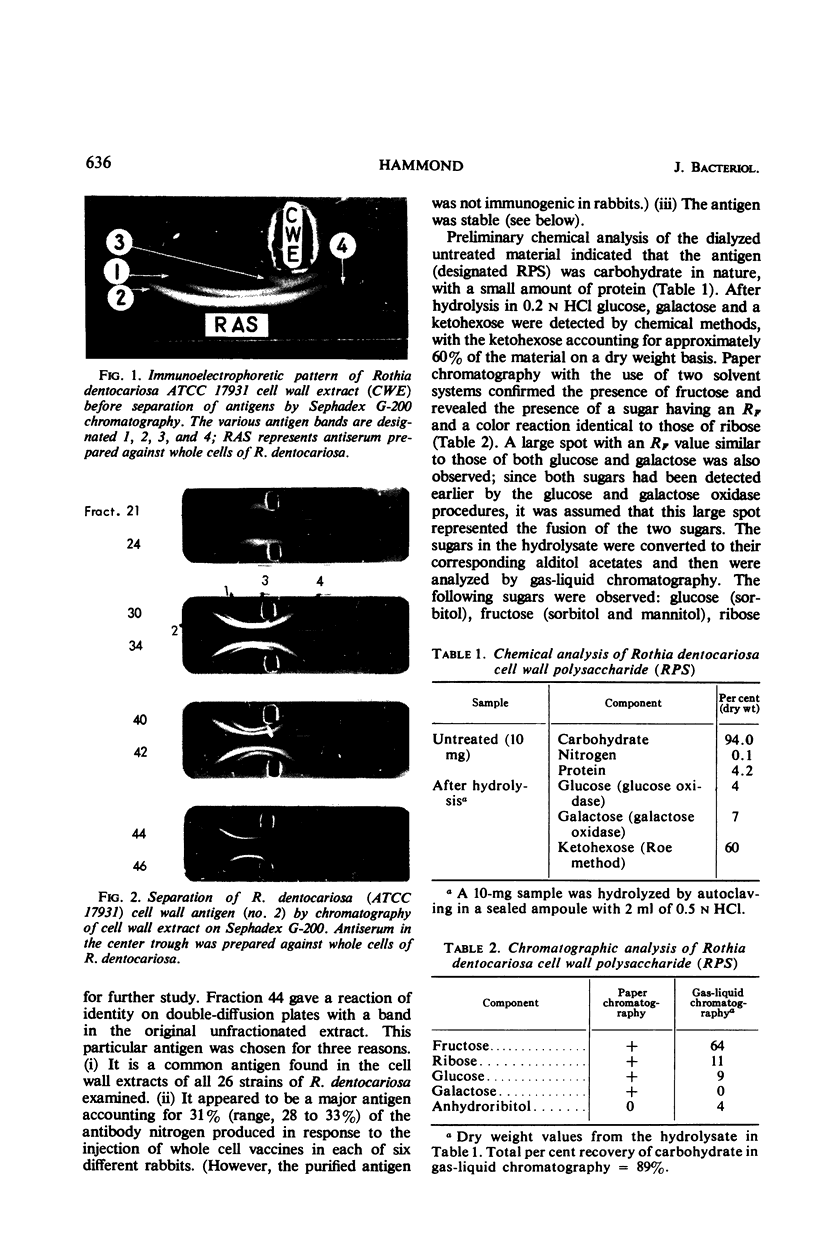

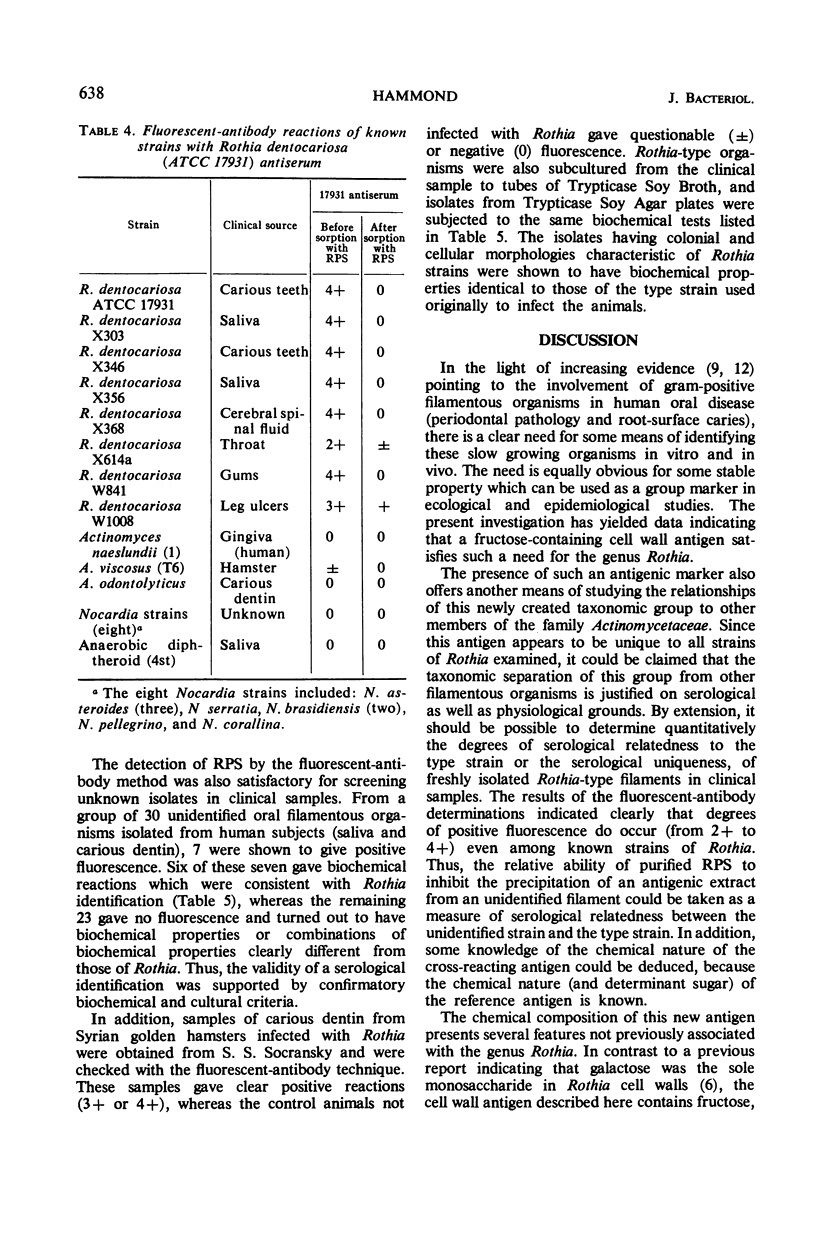
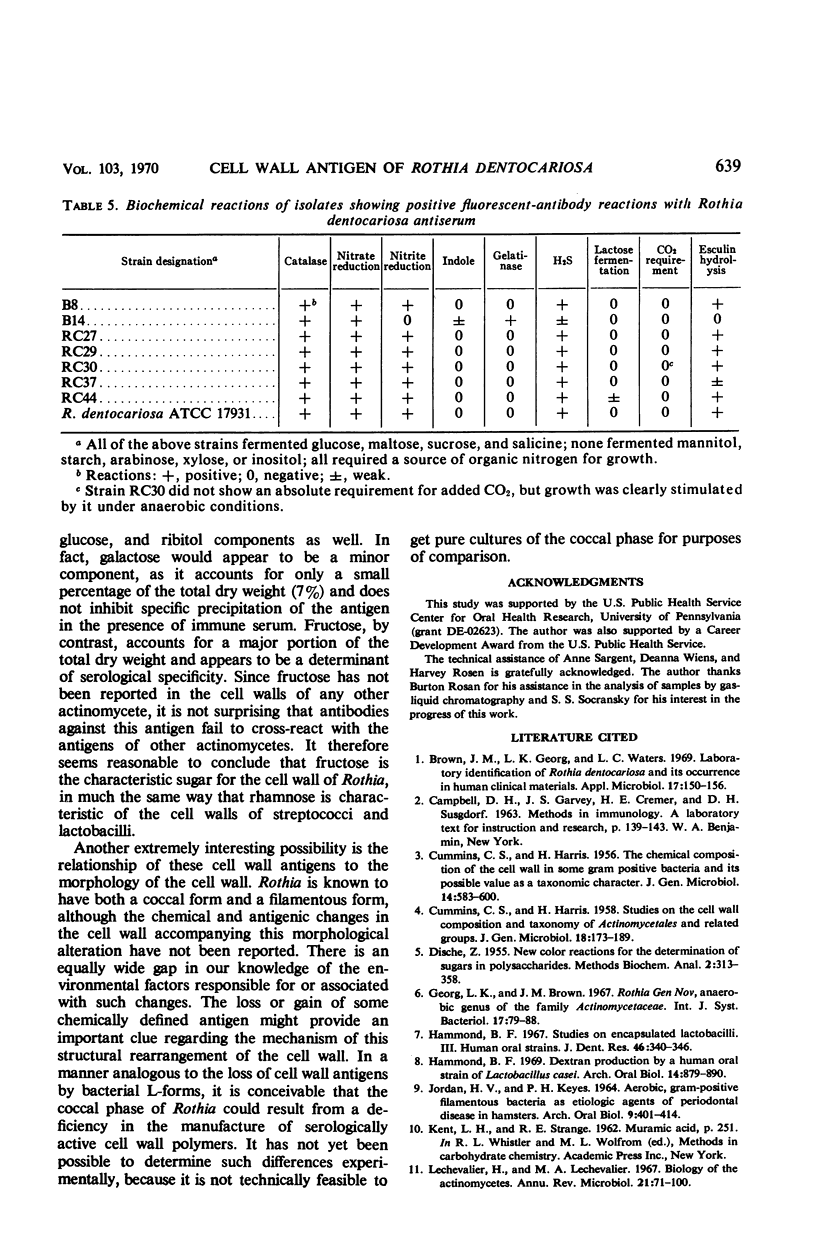

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. M., Georg L. K., Waters L. C. Laboratory identification of Rothia dentocariosa and its occurrence in human clinical materials. Appl Microbiol. 1969 Jan;17(1):150–156. doi: 10.1128/am.17.1.150-156.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. Studies on the cell-wall composition and taxonomy of Actinomycetales and related groups. J Gen Microbiol. 1958 Feb;18(1):173–189. doi: 10.1099/00221287-18-1-173. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- Hammond B. F. Dextran production by a human oral strain of Lactobacillus casei. Arch Oral Biol. 1969 Aug;14(8):879–890. doi: 10.1016/0003-9969(69)90266-0. [DOI] [PubMed] [Google Scholar]
- Hammond B. F. Studies on encapsulated lactobacilli. 3. Human oral strains. J Dent Res. 1967 Mar-Apr;46(2):340–346. doi: 10.1177/00220345670460020501. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Lechevalier H. A., Lechevalier M. P. Biology of actinomycetes. Annu Rev Microbiol. 1967;21:71–100. doi: 10.1146/annurev.mi.21.100167.000443. [DOI] [PubMed] [Google Scholar]
- Montague E. A., Knox K. W. Antigenic components of the cell wall of Streptococcus salivarius. J Gen Microbiol. 1968 Dec;54(2):237–246. doi: 10.1099/00221287-54-2-237. [DOI] [PubMed] [Google Scholar]
- Roth G. D., Flanagan V. The pathogenicity of Rothia dentocariosa inoculated into mice. J Dent Res. 1969 Sep-Oct;48(5):957–958. doi: 10.1177/00220345690480056101. [DOI] [PubMed] [Google Scholar]
- Ullmann W. W., Cameron J. A. Immunochemistry of the cell walls of Listeria monocytogenes. J Bacteriol. 1969 May;98(2):486–493. doi: 10.1128/jb.98.2.486-493.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]




