Abstract
The presence of large-amplitude, slow waves in the EEG is a primary characteristic that distinguishes cerebral activity during sleep from that which occurs during wakefulness. Although sleep-active neurons have been identified in other brain areas, neurons that are specifically activated during slow-wave sleep have not previously been described in the cerebral cortex. We have identified a population of cells in the cortex that is activated during sleep in three mammalian species. These cortical neurons are a subset of GABAergic interneurons that express neuronal NOS (nNOS). Because Fos expression in these sleep-active, nNOS-immunoreactive (nNOS-ir) neurons parallels changes in the intensity of slow-wave activity in the EEG, and these neurons are innvervated by neurotransmitter systems previously implicated in sleep/wake control, cortical nNOS-ir neurons may be part of the neurobiological substrate that underlies homeostatic sleep regulation.
Keywords: Fos, interneuron, neuronal NOS, sleep deprivation, wakefulness
Sleep is well known to have recuperative properties (1) and to facilitate performance of learned behaviors (2, 3); conversely, sleep deprivation results in cognitive and performance deficits (4). The presence of large-amplitude, slow waves in the EEG is the hallmark that distinguishes cerebral activity during sleep from wakefulness. Because the intensity of slow waves measured in the delta range of the EEG (1.0–4.0 Hz) appears to be homeostatically regulated and proportional to prior wake duration (5), slow-wave activity (SWA) has been hypothesized to be associated with the restorative function of sleep and with synaptic plasticity (2, 3, 6).
The neural circuitry underlying SWA involves a corticothalamocortical loop and interplay between a hyperpolarization-activated cation current (Ih) and a low-threshold Ca2+ current (It) in thalamocortical neurons (7, 8). It is currently unknown whether the cerebral cortex is an active participant or simply a passive conveyor of sleep history-dependent SWA dynamics. Identification of a discrete sleep-active population of cortical neurons, such as those in the basal forebrain (BF) (9) and the preoptic-anterior hypothalamus (POAH) (10–13), would help distinguish between these alternatives.
In experiments conducted in three mammalian species, we found that GABAergic interneurons expressing the enzyme neuronal NOS (nNOS) are activated both during spontaneous sleep and during recovery sleep (RS) after sleep deprivation (SD). The proportion of nNOS-immunoreactive (nNOS-ir) neurons that are activated during sleep is correlated with a measure of SWA intensity known as “delta energy” (14, 15). Because sleep-active, nNOS-ir neurons are innervated by neurotransmitter systems previously implicated in sleep/wake control, cortical nNOS-ir neurons may be a previously unrecognized cell type involved in SWA and part of the neurobiological substrate that underlies homeostatic sleep regulation.
Results
Fos Expression Is Increased in Cortical nNOS Neurons During RS.
Fos expression has previously been used to identify sleep-active brain areas within the POAH, specifically, the ventrolateral (10) and median (12) preoptic areas. We used this methodology in conjunction with immunolabeling for phenotypic markers to identify specific neuronal populations that are active during sleep in three rodent species [see supporting information (SI) Fig. S1 for a schematic of the experimental protocols]. In the course of these studies, we observed that a population of cerebral cortex neurons which express nNOS showed greatly elevated Fos expression during RS after a period of SD. These “sleep-active” nNOS-ir neurons were intensely stained, typically had a bipolar or multipolar shape, were ≈10–15 μm in diameter, and were located throughout all cortical layers. These anatomical features of nNOS-ir neurons (Fig. 1D) are very similar to those reported (16, 17). We also observed a large number of weakly stained nNOS cells in the cortex. Because immunostaining of weakly stained cells did not reliably label cellular processes, and thus did not allow unequivocal determination of immunostained neurons from background signal, further analyses were performed only on intensely stained nNOS-ir cells.
Fig. 1.
Identification of sleep-active neurons in the rat cerebral cortex. (A) During the 2.5-h RS period that followed 6-h SD, wakefulness was significantly reduced (P < 0.05). (B) EEG SWA during NREM sleep was elevated for ≈2 h after the end of SD compared with the baseline level recorded 24 h earlier in the same animals. (C) nNOS-ir neurons (red cytoplasm) in the cerebral cortex did not express Fos (black nuclei) in the SD group (a) but expressed Fos in the RS group (b). (D) Schematic showing Fos+/nNOS-ir neuron distribution in two representative brain sections. Blue circles represent single-stained nNOS-ir neurons and orange squares represent double-stained Fos+/nNOS-ir neurons. Few double-stained neurons are found in the cortex after SD, whereas many double-stained neurons are found in the cortex after RS. (E) The proportion of Fos+/nNOS-ir cells was significantly larger in the RS group than in the SD group in every cortical region and in the caudate-putamen. Data are mean ± SEM. *, P < 0.05 compared with corresponding SD group. Abbreviations: Cg Cx, cingulate cortex; M Cx, motor cortex; S Cx, somatosensory cortex; I Cx, insular cortex; Pir Cx, piriform cortex; CPu, caudate-putamen; PFA, perifornical area.
As indicated in Fig. 1 and Fig. S2, the number of Fos+/nNOS-ir neurons in the rat cortex is greatly increased during RS, a period typically associated with reduced wakefulness, increased total sleep time (Fig. 1A), and increased SWA during nonrapid eye movement (NREM) sleep (Fig. 1B). Because nNOS-ir neurons are found in multiple cortical regions and in other brain areas, we evaluated the regional variation of Fos expression in nNOS-ir neurons. Fos+/nNOS-ir double-labeled cells were found in multiple cortical regions and in multiple cortical layers during RS, whereas very few double-labeled cells were observed during SD (Fig. 1 C and D). Fig. 1E demonstrates the significant increase in Fos+/nNOS-ir cells in all cortical regions examined. The proportion of double-labeled cells also increased during RS in the caudate-putamen, however, the proportion was smaller in this brain region than in any cortical area examined. The percentage of double-labeled cells did not differ between RS and SD in the perifornical area of the hypothalamus (Fig. 1E), a region in which wake-active cells are located.
To evaluate the generality of the increase in Fos expression in nNOS-ir cells during RS, we performed a similar experiment in mice. The majority of nNOS-ir neurons were concentrated in layers V and VI in the mouse cortex rather than being more diffusely distributed among cortical layers as in the rat. Nonetheless, an increased proportion of Fos+/nNOS-ir neurons was found during RS relative to wakefulness in all eight cortical areas examined (Fig. 2). In the mouse caudate-putamen, the percentage of double-labeled cells was significantly higher in the posterior part during RS, but not in the anterior part (Fig. 2C).
Fig. 2.
Fos expression in nNOS-ir neurons in mice that were sleep deprived for 6 h or allowed to sleep for 2.5 h after the SD in Experiment 2. (A) In the cerebral cortex, most nNOS neurons did not express Fos during SD (a) but expressed Fos during RS (b). (B) Schematic drawing of Fos/nNOS neuron distribution in representative brain sections at seven levels relative to bregma. Blue circles represent single-stained nNOS neurons and orange squares represent double-stained Fos/nNOS neurons. Few double-stained neurons are found in the cortex after SD (Upper), whereas many double-stained neurons are found in the cortex after RS (Lower). (C) Quantitative analysis demonstrates a significantly greater percentage of Fos-ir cells in all examined regions of the cortex and in the posterior part of the caudate-putamen in mice during RS after SD than during SD itself. *, P < 0.05 compared with corresponding group of sleep-deprived animals. Abbreviations: Cg Cx, cingulate cortex; M Cx, motor cortex; S Cx, somatosensory cortex; I Cx, insular cortex; Pir Cx, piriform cortex; Ent Cx, entorhinal cortex; TeA Cx, TA cortex; V Cx, visual cortex; CPu, a, caudate-putamen, anterior (+1.0 mm from bregma); CPu, p, caudate-putamen, posterior (−1.5 mm from bregma).
Fos Expression Is Increased in Cortical nNOS Neurons During Spontaneous Sleep.
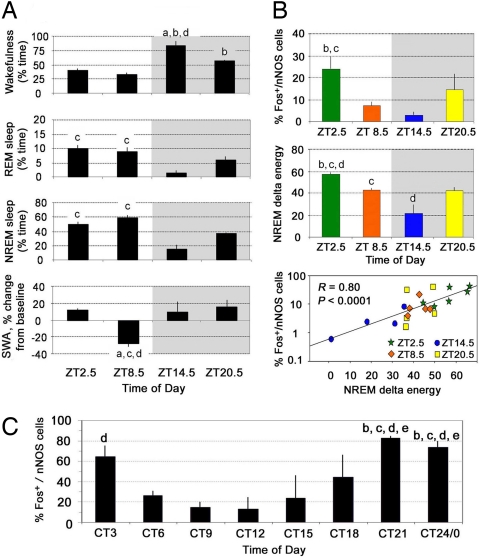
The above results indicate that nNOS-ir neurons in the cortex are activated during RS when SWA is high, but do not address whether nNOS-ir neurons are active during spontaneous sleep. To determine the relationship between various sleep parameters and nNOS cell activation, we measured Fos expression in nNOS-ir neurons in spontaneously sleeping mice. Although the amounts of both NREM and REM sleep were similar in mice during the 2.5 h before death at ZT2.5 and ZT8.5 (Fig. 3A), the proportion of Fos+/nNOS double-labeled cells was significantly higher in mice killed at ZT2.5 when SWA was high, than at ZT8.5 when SWA was low (Fig. 3B). On the other hand, the proportion of Fos+/nNOS double-labeled cells was low at ZT14.5 (Fig. 3B) when the few bouts of NREM sleep interspersed between sustained waking periods were characterized by high levels of SWA relative to baseline (Fig. 3A), so the correlation with SWA was not perfect. Because Fos expression depends on Ca2+-dependent phosphorylation of CREB, we reasoned that a sustained period of NREM sleep with high SWA might be necessary for intracellular Ca2+ to accumulate in nNOS neurons and for transcription and subsequent translation of Fos to be initiated. NREM delta energy is a parameter that integrates both SWA and NREM sleep time (14, 15). The proportion of Fos+/nNOS double-labeled cells was highly correlated with NREM delta energy during the 2.5 h before the animals were killed (R = 0.61, P < 0.005), more highly correlated than with either NREM sleep time (R = 0.33, P = 0.156) or SWA (R = 0.35, P = 0.125) alone. Assessment of different regression models identified the exponential y = 1−e−bx as the best fit (R = 0.80, P < 0.0001) for the relationship between NREM delta energy and the proportion of Fos+/nNOS cortical neurons, indicating a nonlinear, asymptotic relationship between these parameters (Fig. 3B). The number of nNOS-ir neurons did not change across the 24-h period (Fig. S3).
Fig. 3.
Sleep parameters and Fos expression in nNOS-ir neurons of mouse and hamster cortex during spontaneous sleep and wakefulness. (A) Mice were killed at four time points across the light/dark cycle; sleep/wake amounts and EEG SWA levels were calculated for the 2.5-h period before they were killed. Statistical comparisons were made among all groups; letters at the top of bars indicate significant differences (P < 0.05) compared with the time bins indicated [from left to right: a–d in mice (A and B) and a–h in hamsters (C)]. Thus, the amount of wakefulness was significantly higher in the dark period (shaded) at ZT14.5 than during the light period (nonshaded) at ZT2.5 or ZT8.5. Conversely, the amounts of NREM and REM sleep were significantly higher during the light (ZT2.5 and ZT8.5) than during the dark at ZT14.5. SWA was significantly lower at ZT8.5 than at ZT2.5, 14.5, or 20.5. (B) Although sleep amounts were similar at ZT2.5 and ZT8.5, the percentage of Fos+/nNOS-ir cells was significantly higher at ZT2.5 than at ZT8.5 and thus paralleled the high levels of SWA. Across all groups, the percentage of Fos+/nNOS-ir cells in the mouse cortex was highly correlated with NREM delta energy (the relationship is presented here as a semilog plot). (C) In hamsters killed after exposure to constant light for at least 2 weeks, the profile of Fos expression in nNOS-ir cells was similar to that in mice: The highest percentage of Fos+/nNOS-ir cells was found at CT3 during the inactive phase when EEG SWA is high and the lowest proportion at CT9–12 when EEG SWA is low. During the active phase, the proportion of Fos+/nNOS-ir cells is low at CT15 and increases because sleep debt accumulates late in the active phase (CT21–24). Data are shown as mean ± SEM.
A similar profile of Fos expression in nNOS-ir neurons was observed in a third mammal, the golden hamster (Mesocricetus auratus). In this experiment, EEG was not measured, but hamsters were provided with running wheels to record individual locomotor activity rhythms and killed at different phases of the circadian cycle after a minimum of 2 weeks in constant light (LL; 200–300 lux). The proportion of Fos+/nNOS-ir neurons was very high early in the inactive phase (CT3), when high levels of NREM sleep and SWA have been described in the golden hamster (18), and decreased across the inactive phase with the lowest proportion of double-labeled cells observed early in the “subjective night” (i.e., the active phase; CT12–CT15 in Fig. 3C). Although we did not record EEG in this experiment, this temporal profile of Fos+/nNOS-ir neurons can be expected to correlate with NREM delta energy; both NREM sleep and SWA are at their highest levels early during the inactive phase, and SWA exponentially decays during subsequent sleep in the inactive phase in this species (18). A significantly higher proportion of Fos+/nNOS-ir double-labeled cells was also observed in the hamster cortex during the latter half of the active phase (CT21 to CT0). Higher NREM delta energy might be expected at this time because longer sleep episodes occur late in the subjective night, since a sleep debt will have accumulated consequent to prolonged wakefulness bouts that occur earlier in the active period.
Fos+/nNOS Neurons Appear to Be a Unique Population of Sleep-Active Cortical Interneurons.
nNOS-ir neurons are the smallest currently known subdivision of GABAergic projection neurons in the mouse cortex. The next larger subdivision are neurons that express neuropeptide Y (NPY) (19). Therefore, we conducted triple-labeling experiments of Fos, nNOS, and NPY in mouse cortex and found that Fos was expressed in neurons that stained for both nNOS and NPY, but was not found in neurons that stained only for NPY (Fig. 4A).
Fig. 4.
Immunohistochemical characterization of sleep-active nNOS-ir cells. (A) Triple staining for nNOS (a), NPY (b), and Fos (c) immunoreactivity in mouse cortex during RS. Neurons that express both nNOS and NPY contain Fos (arrows), but neurons that express only NPY do not contain Fos (triangles). (B) Double staining for calretinin (triangles) and Fos (arrow) immunoreactivity in mouse cortex indicating that few calretinin-ir neurons express Fos during RS.
To determine whether Fos is expressed in the cerebral cortex in other interneuron populations during RS, we stained for Fos and the calcium binding protein calretinin (Fig. 4B). As described (20), calretinin-ir neurons were present in all cortical layers but were more densely distributed in layers II/III. Some calretinin-ir neurons are known to express nNOS (21), but the absolute number of calretinin-ir neurons in the cortex (22) is much higher than the number of nNOS-ir neurons (Fig. 2) (19). In contrast to nNOS-ir cells, Fos expression in calretinin-ir neurons was very low during RS (<2% of the total number of calretinin-ir neurons throughout the cortex). Given the similar response of nNOS-ir neurons to RS among cortical regions (Figs. 1 and 2), these results suggest that nNOS is a unique neurochemical marker for a sleep-active cell population in the cerebral cortex.
Discussion
Using the immediate early gene Fos as a marker of neuronal activity (23), we have identified a cell type in the cortex in which the expression of Fos is induced during both spontaneous sleep and during RS after a period of SD. Fos expression in these neurons parallels SWA in three mammalian species but is most highly correlated with NREM sleep delta energy. The presence of sleep-active cells in the cerebral cortex was suggested by electrophysiological studies as early as 1964 (24), but the phenotype of these “nonpyramidal tract neurons” has been unknown. Thus, the present study identifies nNOS as a phenotypic marker of cortical neurons that are likely to be sleep-active.
Since its introduction 20 years ago (25, 26), Fos immunohistochemistry has become widely used in neuroscience as a marker of functional activity. Increased neuronal firing is typically accompanied by calcium influx and/or an increase in intracellular cAMP, leading to the phosphorylation of CREB, CRE-mediated c-fos transcription, and ultimately, translation of Fos protein (23). Although this cascade provides the basis for using Fos as a functional marker, Fos can also be induced under conditions that are unrelated to changes in neuronal firing, such as during development, after seizures, hypoxemia or toxin treatment, and after lesions (23). In previous studies, Fos expression has been found to correspond well with the patterns of neuronal firing during sleep and wakefulness in the hypothalamus (10, 27) and brainstem (28, 29). Although the results of the present study are suggestive that cortical nNOS neurons may increase firing during sleep, it is possible that activation of Fos in nNOS neurons could reflect the well established role of nitric oxide in the regulation of blood flow. Cellular electrophysiological studies will be needed to determine the firing pattern of nNOS neurons across arousal states.
To this point, sleep-active neurons have only been found in the preoptic area and BF (10), although a poorly defined region in the vicinity of the nucleus tractus solitarius has also been found to be sleep-active (30–32). The sleep-active neurons in ventrolateral (10) and median (12) preoptic areas are known to be GABAergic and thus thought to play important regulatory roles in sleep by inhibiting wake-promoting, neuronal populations in the perifornical and posterior hypothalamus (10–13). Because the cerebral cortex is not typically thought to be a region involved in the regulatory control of sleep and wakefulness, the function of a sleep-active neuronal population in the cortex is not immediately clear. Given the correlation with NREM delta energy (Fig. 3B), a parameter that integrates both NREM sleep amount and SWA (15), attention is immediately focused on the slow waves that occur during NREM sleep.
SWA, the slow oscillation in the delta range of the EEG, is thought to be essential for the restorative function of sleep (1–6). SWA may facilitate synaptic consolidation or produce synaptic downscaling, thereby increasing signal-to-noise ratios in relevant neural circuits (6). Slow waves originate from corticothalamic feedback loops that bring subsets of cortical neurons into mutually synchronous firing and pausing states (7, 8); the greater the number of neurons participating in a synchronous ensemble, the larger the amplitude of the delta wave in the EEG (7). However, the neuronal mechanisms of EEG coherence over large cortical areas are less clear. Because GABAergic nNOS-ir neurons send long-range projections within the cortex (19, 33), form exclusively axodendritic connections with other neurons, and probably widely arborize in the layers in which their cell bodies are located (34), nNOS-ir neurons appear to be well suited to synchronize cortical EEG activity over large brain areas. In this regard, it is noteworthy that nNOS-ir cell bodies and their proximal dendrites receive both cholinergic (35) and serotonin inputs (36), neurotransmitters that have historically been implicated in arousal state control.
Pharmacological studies have previously implicated nitric oxide and inducible NOS in sleep (37, 38). However, the anatomical focus of these studies has been the BF rather than the cerebral cortex. Extracellular adenosine is known to accumulate in the BF with prolonged wakefulness (39, 40). Prolonged waking activates inducible NOS in the BF which can cause adenosine release and thereby facilitate sleep (37). From the current studies, it is uncertain whether activation of nNOS-ir cortical neurons during sleep is related to nNOS itself, or whether nNOS is simply a phenotypic marker for this sleep-active cell type. Because nitric oxide is involved in short-term dynamic variations of the strength of synapses of cortical networks (41) and levels of SWA differ between nNOS knockout and WT mice throughout the day (42), production of nitric oxide by cortical nNOS may indeed have a significant role in synchronization of cortical EEG activity.
Because the present results are correlative, we cannot determine whether activation of nNOS neurons is necessary for SWA generation or whether activation of nNOS neurons is secondary to high SWA or delta energy in the cortex. Selective lesions or manipulation of neuronal activity of cortical nNOS neurons will be necessary to evaluate a potential causal relationship between activity of nNOS cells and SWA generation. However, given the apparent activation of the nNOS-ir neurons during sleep in multiple species, the correlation of this activation with NREM delta energy, and the innervation of these cells by neurotransmitter systems previously thought to be involved in the sleep-wake regulation, cortical nNOS-ir neurons may be a previously unrecognized cellular component involved in SWA and/or part of the neurobiological substrate of homeostatic sleep regulation. Dysfunction of nNOS-ir neurons or their activation during sleep may have implications for sleep disorders such as insomnia and, given the influence of sleep on mood (43), memory (2), and cortical plasticity (2), possibly other neurological and psychiatric disorders involving the cerebral cortex.
Methods
All studies were conducted in accordance with the principles and procedures described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committees at SRI International and the University of Washington.
Experiment 1: SD and RS in Rats.
Twelve male Sprague–Dawley rats (350–450 g) were housed in separate cages in a room with a 12 h dark/12 h light cycle, with lights on at 07:00. The temperature in the room was maintained at 21–23°C. Food and water were provided ad libitum. No sooner than 1 week after surgery, the rats were connected for EEG and electromyography (EMG) recording (SI Methods, Experiment 1). Based on the visual observations and inspection of the EEG and EMG, rats were kept awake for 6 h (SD group: ZT2.5–ZT8.5; RS group: ZT0–ZT6) by lightly tapping the cage or introducing novel objects into the cage. At the end of the 6-h SD period, the SD group (n = 6) was administered an overdose of pentobarbital (150 mg/kg ip) and perfused transcardially with 100 ml of PBS, followed by 400 ml of phosphate-buffered 10% formalin (Sigma–Aldrich). The RS group (n = 6) was allowed to sleep undisturbed for 2.5 h and then perfused according to the same procedure. All rats were perfused within an interval of 30 min so that the median time of perfusion was ZT8.5 for both groups.
Experiment 2: SD and RS in Mice.
Male C57BL/6 mice (n = 12), aged 8 weeks, were maintained as described for the rats in Experiment 1. On the experimental days, mice were divided into two groups (SD and RS) and treated as described for rats in Experiment 1, except they were perfused transcardially with 20 ml of PBS followed by 20 ml of phosphate-buffered 10% formalin.
Experiment 3: Spontaneous Sleep/Wake in Mice.
After a 14 d (minimum) post-surgery recovery period (SI Methods, Experiment 3), male C57BL/6 mice (n = 24) were placed in the recording environment with ad libitum food and water and maintained in an LD12:12 cycle throughout experimentation. Recording cables (Pinnacle Technologies) were attached to the cranial implants for at least 48 h before initiation of recordings. Digitized EEG (digitization rate 100 Hz) and EMG (digitization rate 200 Hz) were collected via the Embla 16-channel A10 hardware system and visualized and stored with the Somnologica Science software application. After baseline recording for a minimum of 24 h, mice were killed by an overdose of pentobarbital at ZT 2.5, 8.5, 14.5, or 20.5 (n = 6 for each time group). All mice were perfused within a 30-min interval so that the median time of perfusion was ZT 2.5, 8.5, 14.5, or 20.5. Brains were removed and processed for Fos/nNOS immunohistochemical staining. Perfusions and histochemical procedures were as described in Experiment 2.
Sleep state classification and EEG spectral analysis were performed with the SleepSign for Animal software application (Kissei Comtec). EEG data were band-pass filtered (1–30 Hz), and EMG data were high-pass filtered (>10 Hz) for ease of visualization. Vigilance states were classified in 10-s epochs as wake, REM, or NREM sleep by individuals expert in rodent sleep state classification as described for rats in Experiment 1. EEG data were fast Fourier transformed in 10-s epochs. The average delta power (1–4 Hz) was summed for each NREM sleep epoch and averaged over all NREM sleep epochs. Baseline level of delta power was determined as an average of values calculated during the following four time intervals: ZT0–ZT2.5, ZT6–ZT8.5, ZT12–ZT14.5, and ZT18–ZT20.5. To reduce within-group variability, the delta-power measures for each mouse were normalized by dividing them by the baseline level for that mouse. Sleep states as a percentage of time, NREM sleep delta power, and NREM delta energy were calculated from the 2.5 h preceding the animals' being killed. Whereas delta-power measurements are considered a measure of sleep intensity, NREM delta energy (delta power summed over all NREM epochs) is a measure that takes both NREM time and sleep intensity (SWA) into account (14, 15).
Experiment 4: Spontaneous Rest-Activity in Hamsters.
Male golden hamsters (M. auratus) (Charles River) were individually housed and provided with running wheels. Locomotor activity rhythm data were collected and analyzed with ClockLab (Actimetrics). After 1 week under LD14:10 light/dark cycle, hamsters were released in constant dim light (LL; 200–300 lux). After a minimum of 2 weeks in LL, hamsters were killed at one of eight phases of the circadian cycle (CT3: n = 3; CT6: n = 5; CT9: n = 3; CT12: n = 4; CT15: n = 3; CT18: n = 3; CT21: n = 3; CT24/0: n = 4). Hamsters were deeply anesthetized with pentobarbital (20 mg per 100 g of body weight; i.p.) and perfused with 25 ml of heparinized 0.01 M PBS followed by 100 ml of cold 4% paraformaldehyde in 0.1M phosphate buffer. Brains were postfixed overnight at 4°C.
Histochemical Techniques.
Brains were removed, fixed for 4 h in formalin, equilibrated in PBS with 30% sucrose, and stored at 4°C. For rats, coronal sections encompassing the area between 0 mm and −5 mm from bregma were cut at 40 μm on a freezing microtome. For mice, the entire brain was cut at 40 μm. Hamster brains were cut at 40 μM on a cryostat.
Brain sections were treated with 1% H2O2 for 15 min to quench endogenous peroxidases and then incubated overnight in rabbit-anti-cFos antisera (1:15,000; Calbiochem) at room temperature. Tissue was then rinsed in PBS, incubated in biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch) for 2 h at room temperature, incubated with peroxidase-conjugated avidin–biotin complex (ABC; Vector Laboratories) for 1 h, and followed by the addition of 0.05% diaminobenzidine tetrahydrochloride and 0.01% H2O2 with 1% NiSO4 to produce a black reaction product in cell nuclei. The sections were then incubated in mouse anti-nNOS monoclonal antibody (1:5,000; Sigma-Aldrich). Specificity of this nNOS monoclonal has been described (44, 45). Tissue was rinsed in PBS, incubated in biotinylated donkey anti-mouse IgG (1:500; Jackson ImmunoResearch) for 1 h at room temperature, incubated with biotin-conjugated alkaline phosphatase (ABC-AP) for 2 h (Vector Laboratories), washed again, and reacted in a working solution of Vector Red substrate (VectorR Red AP Substrate Kit I; Vector Laboratories) to produce a red reaction product. All tissue was mounted on gelatin-coated slides, dehydrated in ascending concentrations of ethanol, delipidated in xylenes, and coverslipped. Dilutions of all antibodies was done in 5% donkey serum (Jackson ImmunoResearch), PBS, and 1% Triton X-100. Fos/calretinin double staining was done according to a similar protocol by using rabbit anti-calretinin antibody (1:1,000; Fisher Scientific), except that diaminobenzidine tetrahydrochloride without NiSO4 was used to produce the second label (brown staining).
In the triple-label study, mouse brain sections were first stained for Fos by using the ABC/DAB-Ni method and then processed for nNOS/NPY double-fluorescence labeling. Mouse brain sections were incubated overnight in mouse anti-nNOS (1:5,000; Sigma-Aldrich) antibody at room temperature. Tissue was rinsed in PBS, incubated in biotinylated donkey anti-mouse IgG, rinsed in PBS, and incubated in Alexa Fluor 594 streptavidin conjugate (1:500; Invitrogen). Tissue was again rinsed in PBS and incubated in the rabbit anti-NPY (1:1,000) antibody. Tissue was rinsed in PBS and incubated in Alexa Fluor 488 donkey anti-rabbit IgG (1:500; Invitrogen) for 2 h at room temperature. Tissue was then mounted on gelatin-coated slides and coverslipped by using Fluoromount mounting media (Electron Microscopy Sciences).
Cell Counts.
Brain sections were examined under light microscopy (Leica DM5000B) equipped with a CCD video camera operating with a computer-based, anatomical-mapping and image-analysis system (StereoInvestigator; Microbrightfield). A single examiner, blind to treatment conditions, performed cell counts. The regions for nNOS cell counting were selected by outlining the cortex and caudate-putamen bilaterally at low power magnification (×5 objective). For the lateral hypothalamus, the specific counting region was mapped by placing a 750 × 500 μm grid dorsal to the fornix at low power magnification (×5 objective). The selected regions were then scanned at high magnification (×20 objective) for the presence of Fos-positive (Fos+) and Fos+/nNOS double-labeled neurons. Neurons were considered to be Fos+ if they contain black Ni+-DAB reaction product that was darker than a visually established threshold. nNOS cells were counted in brain sections at locations −2.0 mm, −2.8 mm, and −3.6 mm from bregma in rats; at the locations +1.0 mm, −1.5 mm, and −3.0 mm from bregma in mice; and at the locations 0 mm, −1.7 mm, and −2.5 mm from bregma in hamsters. The counts were averaged and used for further statistical analysis.
Statistical Methods.
Data were analyzed by using one-way ANOVA and the Student–Newman–Keuls posthoc test. Data were presented as means ± SEM. Differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments.
Research supported by the Sleep Research Society Foundation (D.G.); National Heart, Lung and Blood Institute (T.S.K.); National Institute of Aging (T.S.K.); National Institute of Mental Health (H.O.d.l.I. and T.S.K.); Medical Research Service of the Department of Veterans Affairs (P.J.S.); and Ministry of Education, Culture, Sports, Science and Technology of Japan (T.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.B.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803125105/DCSupplemental.
References
- 1.Borbely A, Achermann P. In: Principles and Practice of Sleep Medicine. Kryger MH, Roth T, Dement WC, editors. Philadelphia: Saunders; 2000. pp. 377–390. [Google Scholar]
- 2.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 3.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 4.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 5.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 6.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 8.McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 9.Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- 10.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 11.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: Effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2079–R2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- 13.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 14.Naylor E, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- 16.Bidmon HJ, et al. Nitric oxide synthase-expressing neurons are area-specifically distributed within the cerebral cortex of the rat. Neuroscience. 1997;81:321–330. doi: 10.1016/s0306-4522(97)00131-0. [DOI] [PubMed] [Google Scholar]
- 17.Fuentealba P, et al. Ivy cells: A population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobler I, Jaggi K. Sleep and EEG spectra in the Syrian hamster (Mesocricetus auratus) under baseline conditions and following sleep deprivation. J Comp Physiol A. 1987;161:449–459. doi: 10.1007/BF00603970. [DOI] [PubMed] [Google Scholar]
- 19.Tomioka R, et al. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21:1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- 20.Park HJ, et al. The distribution and morphology of calbindin. Mol Cells. 2002;14:143–149. [PubMed] [Google Scholar]
- 21.Lee JE, Jeon CJ. Immunocytochemical localization of nitric oxide synthase-containing neurons in mouse and rabbit visual cortex and co-localization with calcium-binding proteins. Mol Cells. 2005;19:408–417. [PubMed] [Google Scholar]
- 22.Tamamaki N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: Are we all ‘fos-ed out’? J Neuroendocrinol. 2002;14:259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- 24.Evarts EV. Temporal patterns of discharge of pyramidal tract neurons during sleep and waking in the monkey. J Neurophysiol. 1964;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- 25.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: Metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 26.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 27.Estabrooke IV, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamuy J, Sampogna S, Morales FR, Chase MH. c-fos Expression in mesopontine noradrenergic and cholinergic neurons of the cat during carbachol-induced active sleep: A double-labeling study. Sleep Res Online. 1998;1:28–40. [PubMed] [Google Scholar]
- 29.Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19:3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnes J, Moruzzi G, Pompeiano O. Synchronization of the E.E.G. produced by low-frequency electrical stimulation of the region of the solitary tract. Arch Ital Biol. 1961;99:33–67. [Google Scholar]
- 31.Eguchi K, Satoh T. Convergence of sleep-wakefulness subsystems onto single neurons in the region of cat's solitary tract nucleus. Arch Ital Biol. 1980;118:331–345. [PubMed] [Google Scholar]
- 32.Eguchi K, Satoh T. Characterization of the neurons in the region of solitary tract nucleus during sleep. Physiol Behav. 1980;24:99–102. doi: 10.1016/0031-9384(80)90020-7. [DOI] [PubMed] [Google Scholar]
- 33.Higo S, Udaka N, Tamamaki N. Long-range GABAergic projection neurons in the cat neocortex. J Comp Neurol. 2007;503:421–431. doi: 10.1002/cne.21395. [DOI] [PubMed] [Google Scholar]
- 34.Seress L, Abraham H, Hajnal A, Lin H, Totterdell S. NOS-positive local circuit neurons are exclusively axo-dendritic cells both in the neo- and archi-cortex of the rat brain. Brain Res. 2005;1056:183–190. doi: 10.1016/j.brainres.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 35.Vaucher E, Linville D, Hamel E. Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience. 1997;79:827–836. doi: 10.1016/s0306-4522(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 36.Cauli B, et al. Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalinchuk AV, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur J Neurosci. 2006;24:1443–1456. doi: 10.1111/j.1460-9568.2006.05019.x. [DOI] [PubMed] [Google Scholar]
- 38.Kalinchuk AV, Lu Y, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem. 2006;99:483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- 39.Porkka-Heiskanen T, et al. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanco-Centurion C, et al. Adenosine and sleep homeostasis in the Basal forebrain. J Neurosci. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kara P, Friedlander MJ. Dynamic modulation of cerebral cortex synaptic function by nitric oxide. Prog Brain Res. 1998;118:183–198. doi: 10.1016/s0079-6123(08)63208-2. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Majde JA, Krueger JM. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 2003;973:214–222. doi: 10.1016/s0006-8993(03)02484-3. [DOI] [PubMed] [Google Scholar]
- 43.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13:505–509. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 44.Szabadits E, et al. Hippocampal GABAergic synapses possess the molecular machinery for retrograde nitric oxide signaling. J Neurosci. 2007;27:8101–8111. doi: 10.1523/JNEUROSCI.1912-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onn SP, Grace AA. Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci. 2000;20:2332–2345. doi: 10.1523/JNEUROSCI.20-06-02332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.