Abstract
Arsenic poisoning affects millions of people worldwide. Human arsenic intake from rice consumption can be substantial because rice is particularly efficient in assimilating arsenic from paddy soils, although the mechanism has not been elucidated. Here we report that two different types of transporters mediate transport of arsenite, the predominant form of arsenic in paddy soil, from the external medium to the xylem. Transporters belonging to the NIP subfamily of aquaporins in rice are permeable to arsenite but not to arsenate. Mutation in OsNIP2;1 (Lsi1, a silicon influx transporter) significantly decreases arsenite uptake. Furthermore, in the rice mutants defective in the silicon efflux transporter Lsi2, arsenite transport to the xylem and accumulation in shoots and grain decreased greatly. Mutation in Lsi2 had a much greater impact on arsenic accumulation in shoots and grain in field-grown rice than Lsi1. Arsenite transport in rice roots therefore shares the same highly efficient pathway as silicon, which explains why rice is efficient in arsenic accumulation. Our results provide insight into the uptake mechanism of arsenite in rice and strategies for reducing arsenic accumulation in grain for enhanced food safety.
Keywords: efflux, influx, arsenic contamination, silicon, aquaporin
Arsenic (As) is a human carcinogen, and there may be no threshold below which it does not cause cancer (1). More than 40 million people worldwide are at risk from drinking As-contaminated groundwater (2), and chronic inorganic As poisoning has reached a massive scale in Bangladesh and West Bengal, India (3). In these countries, As-contaminated groundwater is also widely used for irrigating crops during dry season rice production, adding >1,000 metric tons of As to soil per year in Bangladesh alone and resulting in As accumulation in soils and elevated As uptake by crops (4–6). Elevated As accumulation in rice has the potential to become a new disaster for the population in Southeast Asia (7). As concentrations in rice grain are often high enough to cause concern even in uncontaminated soils containing background levels of As, because paddy rice appears to be particularly efficient in As assimilation compared with other cereal crops (8). Worldwide market surveys show that rice grain contains considerably higher levels of inorganic As than other foods (9, 10). Human intake of As from consumption of rice can be substantial, especially for people who consume a lot of rice (11). It is therefore crucial that the mechanism of As accumulation in rice is understood to counteract this widespread contamination of the food chain.
Plants take up arsenate, the predominant form of As in aerobic soils, through phosphate transporters (12, 13). However, in paddy soils, which are flooded during much of the rice growing season, arsenite becomes the predominant chemical species of As (14). It has been shown that arsenite is taken up via aquaglyceroporins in microbes (13, 15–17). Evidence from physiological studies suggests that arsenite may also be transported by aquaporins in rice (18), although the exact mechanism of arsenite uptake has not been identified in higher plants. Here we report two types of arsenite transporters in rice and their role in As accumulation in rice shoots and grain.
Results and Discussion
NIP Transporters Mediate Arsenite Influx in Rice Roots.
Plant aquaporins transport neutral molecules such as water, glycerol, and urea. These transporters are classified into four major subfamilies based on their homology and localization: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), nodulin 26-like intrinsic membrane proteins (NIPs), and small and basic intrinsic proteins (SIPs) (19). Arsenite, with a pKa of 9.2, is present predominantly as uncharged molecules of arsenous acid [As(OH)3] at pH < 8. Recently, a NIP transporter (Lsi1) for silicic acid, which is also uncharged at pH < 8, has been identified in rice (20). Furthermore, arsenous acid [diameter 4.11 Å; As(OH)3 is a tetrahedron with the largest dimension being between the two neighboring OH groups, modeled using WebLab Viewer, www.marcsaric.de/index.php/WebLab_Viewer_Lite] has a molecular size similar to that of silicic acid (diameter 4.38 Å). Therefore, we hypothesized that arsenite transport is mediated by the transporters for silicic acid.
We determined the transport activity for arsenite in Xenopus oocytes expressing the rice silicic acid transporter Lsi1. Expression of Lsi1 resulted in a 5-fold increase in the arsenite transport activity (Fig. 1A). In the oocytes expressing a mutant lsi1, which has a single mutation at the position of the 132nd amino acid (20), transport activity for arsenite was not significantly different from the control (water injection) (Fig. 1A). Furthermore, Lsi1 was not permeable to arsenate (Fig. 1A). We also assayed the transport activity for arsenite in yeast. Yeast expressing Lsi1 showed 3- to 5-fold-higher transport activity compared with the empty vector control (Fig. 1B). These results indicate that arsenite could be transported by the silicic acid transporter in rice.
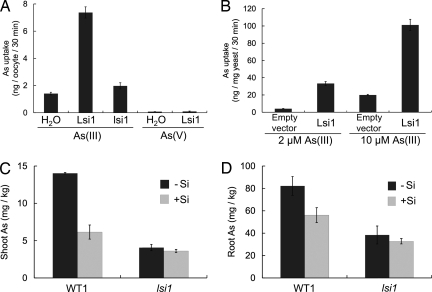
Fig. 1.
Arsenite transport activity of Lsi1 in oocytes, yeast, and rice. (A) As uptake by oocytes expressing Lsi1 or mutant lsi1, with H2O as the control. Oocytes were exposed to 100 μM arsenite or arsenate for 30 min. (B) As uptake by the yeast mutant (acr3) expressing Lsi1 or the empty vector. Yeast was exposed to 2 or 10 μM arsenite for 30 min. (C and D) Concentration of As in shoots (C) and roots (D) of the wild-type rice (WT1 = cv. Oochikara) and the lsi1 mutant. Two-week-old seedlings were exposed to a nutrient solution containing 2 μM arsenite with or without 0.5 mM silicic acid for 1 day. Data are means ± SD (n = 3).
We then compared As accumulation between the wild-type rice and a mutant defective in silicon uptake, lsi1 (21). The concentrations of As in the mutant shoots and roots were 71% and 53%, respectively, lower than those of the wild type in the absence of silicic acid after exposure to 2 μM arsenite for 1 day (P < 0.001; Fig. 1 C and D). The addition of silicic acid to the medium significantly (P < 0.01) decreased the As concentrations in both roots and shoots of the wild-type plants but not in the mutant. Because the decreased accumulation of As in the mutant shoots may also result from an effect on As translocation from roots to shoots, we compared short-term (30-min) arsenite uptake by the wild-type and mutant roots. Results showed that As uptake by the mutant roots was 57% lower than that by the wild-type roots [P < 0.01; supporting information (SI) Fig. S1], indicating a role of Lsi1 in arsenite influx to roots. These results are consistent with the oocyte and yeast results (Fig. 1 A and B), confirming that Lsi1 is permeable to arsenite in planta and is responsible for the primary uptake of arsenite from the external solution to the root cells.
There are 10–13 members of the NIP family in the rice genome (22). Functional analyses of NIPs have revealed diverse transport functions for different substrates including glycerol (23), lactic acid (24), urea and formamide (25), boric acid (26), and silicic acid (20). The substrate selectivity of aquaporins is mainly controlled by the ar/R (aromatic/arginine) selectivity filter (27, 28), which is located in the narrowest region on the extramembrane part of the pore. Based on the ar/R regions, NIPs have been subdivided into three groups, NIP I, II, and III (29). To test whether other NIP members can transport arsenite, we expressed three NIP genes belonging to different groups in oocytes. All members tested showed transport activity for arsenite, although the activity varied among them (Fig. 2A). Both Lsi6 (OsNIP2;2) and Lsi1 (OsNIP2;1) belong to NIP III and are permeable to silicic acid, whereas OsNIP1;1 and OsNIP3;1, belonging to NIP I and II, respectively, do not transport silicic acid (29). Arsenous acid is slightly smaller than silicic acid, which may explain why all three NIP groups are permeable to arsenite but only the NIP III members are permeable to silicic acid.
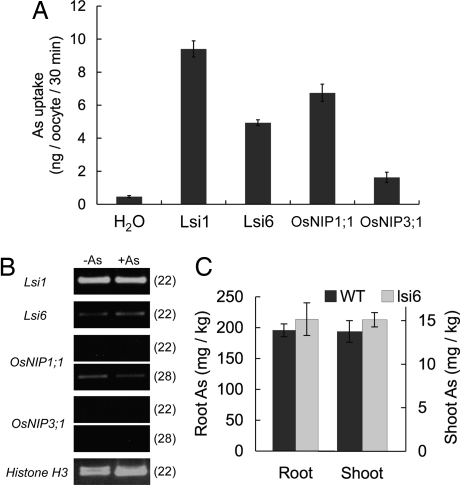
Fig. 2.
Arsenite transport by different NIP transporters. (A) Arsenite transport activity of NIPs. Oocytes expressing different NIPs were exposed to 100 μM arsenite for 30 min. Data are means ± SD (n = 3). (B) Expression level of different NIPs in wild-type rice roots. Seedlings exposed to 0 or 5 μM arsenite for 7 days were used for extraction of RNA. RT-PCR was performed on each sample. Numbers in parentheses are PCR cycles. (C) Concentration of As in the shoots and roots in the wild-type rice (cv. Dongjin) and the T-DNA knockout mutant (lsi6). Seedlings were exposed to 2 μM arsenite for 7 days. Data are means ± SD (n = 3).
We further investigated the expression levels of these NIP genes in the roots of the wild-type rice. Lsi1 showed the most abundant expression among the NIP genes examined, whereas the expression of Lsi6 was much lower than that of Lsi1 (Fig. 2B). The expression of OsNIP1;1 and OsNIP3;1 was very weak. The expression of all genes tested was unaffected by the presence of 5 μM arsenite. Furthermore, knocking out Lsi6 in a T-DNA insertion line of rice had no significant effect on the As concentrations in shoots and roots compared with the wild type (Fig. 2C). This result was further confirmed with a Tos-17 insertion line for Lsi6 (Fig. S2). A short-term (30-min) uptake experiment also revealed that Lsi6 did not play any demonstrable role in arsenite uptake by the roots (Fig. S3). Therefore, although Lsi6 showed transport activity for arsenite when expressed in oocytes, it does not contribute significantly to arsenite uptake by rice roots, probably because of low expression. The expression levels of other rice NIPs have been shown to be very low in the roots (30). Taken together, these data indicate that Lsi1 is the major pathway for arsenite uptake in rice.
An Efflux Transporter for Arsenite in Rice.
Lsi1 is localized at the distal side of both exodermis and endodermis cells of rice roots and mediates the influx of silicic acid—and arsenite according to the present study—into root cells (20). In rice roots, there are two Casparian bands on the exodermis and endodermis, separated by the aerenchyma formed from the destruction of almost all cortex cells except the exodermis and the endodermis. Therefore, to reach the stele, As must be transported sequentially into and out of the exodermis and the endodermis cells. Arsenite is the predominant form of As in rice roots, regardless of whether arsenate or arsenite is supplied to the plants (31), and is therefore the likely form transported into the stele. Recently, an efflux transporter (Lsi2) for silicic acid has been identified from rice roots (32). In contrast to Lsi1, Lsi2 is localized at the proximal side of both exodermis and endodermis cells of rice roots and mediates the efflux of silicic acid from the exodermis and endodermis cells toward the direction of the stele. To investigate whether Lsi2 can transport arsenite, we compared As accumulation between the wild-type rice and a mutant (lsi2) defective in silicic acid transport (32). The As concentration of the mutant shoots was 75% lower than that of the wild-type shoots in the absence of silicic acid (P < 0.001; Fig. 3A). The presence of silicic acid decreased the As concentration in the wild-type shoots significantly (P < 0.001), but not in the mutant. Arsenite was the main As species found in the xylem sap from both the wild type and the lsi2 mutant, and its concentration in the latter was only 9% of the former (P < 0.001; Fig. 3B). In contrast to the lsi1 mutant, there was no significant difference in the short-term (30-min) uptake of arsenite between lsi2 and the wild-type rice (Fig. S4), suggesting that Lsi2 is involved not in the influx of arsenite into roots, but rather in the efflux of arsenite toward the xylem.
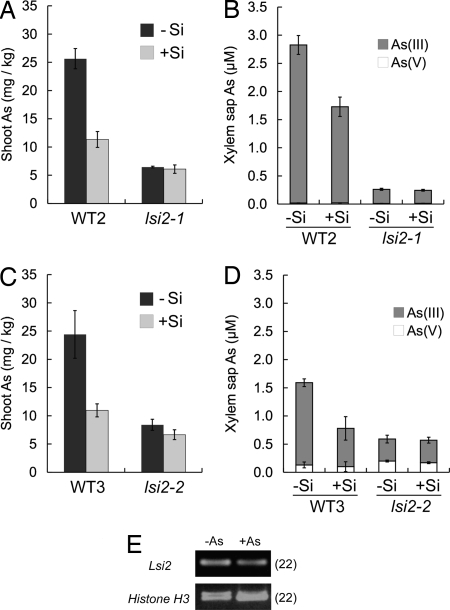
Fig. 3.
As transport and expression of Lsi2. (A and C) The concentration of As in the shoots of the two mutants (lsi2-1 and lsi2-2) and their wild-type rice (WT2 = cv. T-65, WT3 = cv. Koshihikari). Two-week-old seedlings of four lines were exposed to 2 μM arsenite with or without 0.5 mM silicic acid for 7 days. (B and D) The concentrations of arsenite and arsenate in the xylem sap collected from the wild-type rice and lsi2-1 and lsi2-2 mutants after seedlings were exposed to 5 μM arsenite with or without 0.5 mM silicic acid for 1 day. (E) Expression of Lsi2 in rice roots. Two-week-old seedlings of the wild-type rice (cv. Nipponbare) were exposed to 0 or 5 μM arsenite for 7 days, and Lsi2 expression was quantified by RT-PCR. Data in A–D are means ± SD (n = 3).
To examine the efflux transport activity of Lsi2 for arsenite, we expressed Lsi2 in the oocytes, yeast, and Escherichia coli. However, we failed to detect transport activity in these assay systems. Our previous study showed that Lsi2 did not function for silicic acid transport in the yeast assay system but did function in the oocytes. The failure to observe arsenite transport activity in oocytes may result from the toxicity of arsenite that was injected into the cells or the binding of arsenite to thiols and sulfhydryl groups in proteins.
To confirm that Lsi2 is an efflux transporter for arsenite, we took an alternative approach by isolating an allele of lsi2 rice mutant. From the M3 seeds of the rice cultivar Koshihikari irradiated with gamma rays, we obtained a new mutant (lsi2-2) having low silicon uptake (Fig. S5). Comparison of the sequence of Lsi2 between the wild-type rice and the mutant revealed that one nucleotide substitution occurred in the mutant, resulting in one amino acid change at 342 from T to R (Fig. S6). When this mutant was exposed to arsenite, the As concentration in the shoots was 66% lower than that of the wild-type in the absence of silicic acid (P < 0.001; Fig. 3C), and the arsenite concentration in the xylem sap was 73% lower than that of the wild type (P < 0.001; Fig. 3D). Addition of silicic acid to the medium significantly (P < 0.001) decreased both shoot As concentration and xylem sap arsenite concentration in the wild type but not in the mutant (Fig. 3 C and D). The results from the two independent lsi2 mutants indicate that Lsi2 is involved in arsenite transport out of the root cells toward the stele. The expression of Lsi2 in the wild-type roots was abundant and not affected by arsenite exposure (Fig. 3E).
Lsi2 Plays a Crucial Role in Controlling As Concentration in the Rice Grain.
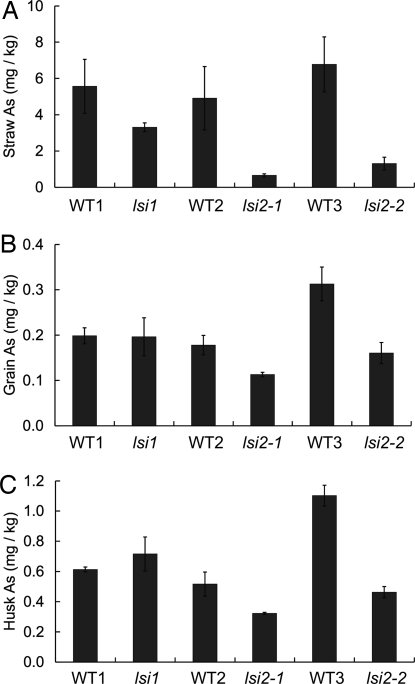
To investigate the role of the Lsi1 and Lsi2 transporters in the accumulation of As in rice gain, we grew the three rice mutants with their corresponding wild-type cultivars in a field experiment on a soil containing a background level of total As (5.3 mg/kg). All three mutants had lower concentrations of As in straw than their wild type, with the difference being particularly pronounced in the two lsi2 mutants, which were only 13–19% of the corresponding wild-type rice (P = 0.06 for the WT1/lsi1 comparison, P < 0.01 for the WT2/lsi2-1 and WT3/lsi2-2 comparisons; Fig. 4A). In the grain and husk, there was no significant difference in the As concentration between the lsi1 and its wild-type rice (Fig. 4 B and C). In contrast, in both lsi2 mutants, the grain As concentration was significantly (P < 0.01) lower than the wild-type rice (Fig. 4B), being 63% and 51%, respectively, of the corresponding wild-type rice. These results indicate that Lsi2 plays a more important role than Lsi1 in As transport to the shoots and ultimately to the grain in rice and strongly suggest that the root-to-shoot translocation is the key step in controlling As accumulation in shoots.
Fig. 4.
The concentrations of As in straw (A), grain (B), and husk (C) of field-grown rice. The three mutants and their wild-type rice were grown in a field in 2007. Data are means ± SD (n = 3).
As transport mechanisms have been studied extensively in bacteria and yeast (33, 34). In microbes, arsenite enters the cell through aquaglyceroporin channels. In the present study, we found that arsenite is transported by aquaglyceroporin NIPs in rice. In bacteria, arsenite is pumped out of the cells by ArsB or ArsAB functioning as an As(OH)3-H+ antiporter or ATP-driven extrusion pump, respectively (33). In yeast, arsenite is transported out of the cells by Acr3p, a plasma membrane-located arsenite efflux transporter (33). Rice Lsi2 has a similarity to E. coli ArsB (18% identity). Although the arsenite transport system in rice appears to be similar to that in bacteria at the cell level, there is a fundamental difference between the two at the organism level: bacteria efflux arsenite for detoxification, whereas arsenite efflux mediated by Lsi2 in rice leads to accumulation in shoots and grain.
In conclusion, for the first time, to our knowledge, in higher plants, we have identified both influx and efflux transporters of arsenite in rice roots, which are involved in arsenite uptake and accumulation. High expression of Lsi1 and Lsi2 in rice leads to a large accumulation of silicon, which benefits yield production (20, 32) but unfortunately also enhances As accumulation in rice shoots and grain. Our results provide important information for developing strategies to reduce As concentration in rice for enhanced food safety. Future research is needed to identify allelic variations in Lsi1 and, particularly, Lsi2 that can favor uptake of silicon over arsenite. At a more practical level, ensuring sufficient silicon availability in the soil is likely to suppress As accumulation in rice.
Materials and Methods
Plant Materials and Growth Conditions.
Three mutants (lsi1, lsi2-1, and lsi2-2) and their corresponding wild-type rice (cv. Oochikara, T-65, and Koshihikari), T2 homozygous seeds of T-DNA insertion line (4A-01373), and T2 homozygous seeds of Tos17 insertion line (NG0012) for Lsi6 were used in the present study. Mutants lsi1 and lsi2-1 were isolated previously (20, 21, 32), and lsi2-2 was isolated as described below. Seedlings were cultured hydroponically as described previously (21). The solution was renewed every 2 days. All experiments were conducted with three replicates in a glasshouse at 25°C with natural light.
As Treatment.
Two-week-old seedlings were exposed to a nutrient solution containing 2 μM arsenite with or without 0.5 mM silicic acid. The treatment solutions were changed once every 2 days, during which period arsenite was stable in the nutrient solution (31). After 1 or 7 days, the roots were washed three times with an ice-cold solution containing 1 mM K2HPO4, 0.5 mM Ca(NO3)2, and 5 mM MES (pH 5.6) to remove apoplastic As and separated from shoots. The samples were dried at 70°C in a oven for 2 days, digested with HNO3, and analyzed for As by using inductively coupled plasma MS (ICP-MS) as described in ref. 31. Analysis of variance was performed to test the significance in the difference between wild types and mutants and between Si treatments.
For the collection of xylem sap, two-week-old rice seedlings were exposed to a nutrient solution containing 5 μM arsenite with or without 0.5 mM silicic acid. After 24 h, stems were cut at 1 cm above the roots. The cut surfaces were rinsed with deionized water and blotted dry. Xylem exudates were collected by micropipette for 1 h. As species in the xylem sap were analyzed immediately by using HPLC-ICP-MS as described in ref. 31.
Transport Activity Assay.
We used both oocyte and yeast expression systems for the determination of arsenite transport activity. Oocytes were isolated from adult female Xenopus laevis frogs and placed in a modified Birth's saline (MBS) solution (88 mM NaCl/1 mM KCl/2.4 mM NaHCO3/15 mM Tris·HCl, pH 7.6/0.3 mM Ca(NO3)2/0.41 mM CaCl2/0.82 mM MgSO4/10 μg·ml−1 sodium penicillin/10 μg·ml−1 streptomycin sulfate). Oocytes were then treated with 0.1% collagenase type B (Roche Diagnostics) in a Ca-free MBS for 1.5 h to remove follicular cell layers, washed five times with MBS free of collagenase, and selected according to the size and development stage. Selected oocytes were incubated for 1 day in MBS at 18°C until the injection of cRNA. The ORFs of different genes including Lsi1, mutant lsi1, Lsi6, OsNIP1;1, and OsNIP3;1, were amplified and cloned as described before (29), and the cRNA with cap analog was synthesized with mMASSAGGE mMACHINE High Yield Capped RNA Transcription Kit (Ambion) according to the manufacturer's instructions. Fifty nanoliters of cRNA (1 ng·nl−1) were injected into the selected oocytes by using a Nanoject II automatic injector (Drummond Scientific). As a negative control, 50 nl of RNase-free water was injected. After incubation in MBS at 18°C for 1 day, the oocytes were exposed to the MBS buffer (pH 7.8) containing 0.1 mM NaAsO2 or 0.1 mM Na2HAsO4′7H2O. After 30 min of exposure, the oocytes were washed five times with MBS without As and homogenized with 500 μl of 0.1 M HNO3. As concentrations were determined by ICP-MS. Experiments were performed with three replicates.
For the transport assay using the yeast expression system, the Lsi1 cDNA was cloned into yeast expression vector pYES2. The arsenite sensitive mutant strain (WΔacr3) was grown at 30°C in a complete YPD medium supplemented with 200 μM G418. Transformation reactions were performed by using S.c.EasyComp Transformation Kit (Invitrogen). One colony was selected in each transformation strain and grown in the liquid YPD medium. The precultured yeast was adjusted to an OD600 value of 2.0. Two or 10 μM arsenite was then added to the medium. After 30 min of incubation with gentle shaking, the culture solutions were washed three times with the YPD solution without arsenite and digested with HNO3. As concentrations were determined by ICP-MS. Each treatment was replicated three times.
Isolation of an Allele of Mutant lsi2-2.
We used M3 seeds of rice (Oryza sativa L. cv. Koshihikari) irradiated with gamma rays for 10 days for isolation of an allele of mutant lsi2. The screening method is based on the tolerance to germanium toxicity (21). We sowed the seeds on a soil containing 50 mg of Ge per kilogram of soil as GeO2. After 1 month of cultivation, we selected seedlings that did not show the Ge toxicity symptoms of brown spots on the leaf blades. We then grew the candidate mutants in a field and performed a second screening by determining silicon uptake. From the candidate mutants, we further amplified ORF of Lsi2 by PCR with primers designed based on the sequence of Lsi2 and sequenced by ABI PRISM 310 Genetic Analyzer using BigDye Terminators V3.1 cycle Sequencing Kit. Finally, we obtained a new mutant (named lsi2-2) that is allelic to lsi2 (named lsi2-1).
RNA Isolation and RT-PCR.
To examine the expression level of different NIPs in rice roots and the effect of arsenite on the gene expression, we exposed the seedlings of a wild-type rice (cv. Nipponbare) to 5 μM arsenite for 7 days. Then, roots were cut and frozen in liquid nitrogen. Total RNA was extracted by using the RNeasy Mini Kit (Qiagen). One microgram of total RNA was used for first-strand cDNA synthesis using a SuperScript II kit (Invitrogen), following the manufacturer's instructions with an oligo(dT)12–18 primer. One-twentieth of the reaction volume was used as template for PCR of Lsi1, Lsi2, Lsi6, OsNIP1;1, and OsNIP3;1, and HistoneH3 was used as an internal control. The reactions were run for 22–28 cycles, depending on genes. The primer sequences for PCR of different genes are as follows: Lsi1, 5′-CGGTGGATGTGATCGGAACCA-3′ (forward) and 5′-CGTCGAACTTGTTGCTCGCCA-3′ (reverse); Lsi6, 5′-GAGTTCGACAACGTCTAATCGC-3′ (forward) and 5′-AGTACACGGTACATGTATACACG-3′ (reverse); OsNIP1;1, 5′-CTGATTGCTGGGCCGATCTCG-3′ (forward) and 5′-GCAGTAGTAGTACTGGCAGTAG-3′ (reverse); OsNIP3;1, 5′-GTTGCAAGAAGAGGAGAGCAAG-3′ (forward) and 5′-CGAAGAAGATGAGGATGAACGTC-3′ (reverse); Lsi2, 5′-ATCTGGGACTTCATGGCCC-3′ (forward) and 5′-ACGTTTGATGCGAGGTTGG-3′ (reverse); HistoneH3, 5′-AGTTTGGTCGCTCTCGATTTCG-3′ (forward) and 5′-TCAACAAGTTGACCACGTCAC G-3′ (reverse).
Field Experiment.
A field trial was carried out in 2007 at the Experimental Farm of Okayama University. The properties of soil used have been described previously (20). Twenty-day-old seedlings of the three mutants and their wild-type rice prepared as described above were transplanted in the field in mid-June and grown under flooded conditions. Each plot (0.7 m × 0.7 m) contained 36 seedlings, and three replicates were included for each line. At the beginning of October, straw and grain were harvested and separated. The grains were dehusked with a small thresher (Kett TR-110). The straw, grain, and husk samples were digested with HNO3 and analyzed for As concentration by ICP-MS.
Supplementary Material
Acknowledgments.
We thank Minoru Nishimura (National Institute of Agrobiological Sciences, Institute of Radiation Breeding, Japan) for providing M3 seeds, the Rice Genome Resource Center for Tos-17 seeds, and Dr. Yaozhong Xu for calculating molecular dimension of arsenous acid. This research was supported by Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation Grant IPG-0006 (to J.F.M.) and Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan 17078008 (to J.F.M.). Rothamsted Research receives grant-aided support from the U.K. Biotechnology and Biological Sciences Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802361105/DCSupplemental.
References
- 1.Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Public health—Arsenic epidemiology and drinking water standards. Science. 2002;296:2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- 2.Nordstrom DK. Public health—Worldwide occurrences of arsenic in ground water. Science. 2002;296:2143–2145. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee Y. A sluggish response to humanity's biggest mass poisoning. Science. 2007;315:1659–1661. doi: 10.1126/science.315.5819.1659. [DOI] [PubMed] [Google Scholar]
- 4.Meharg AA, Rahman M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ Sci Technol. 2003;37:229–234. doi: 10.1021/es0259842. [DOI] [PubMed] [Google Scholar]
- 5.Alam MB, Sattar MA. Assessment of arsenic contamination in soils and waters in some areas of Bangladesh. Water Sci Technol. 2000;42:185–192. [Google Scholar]
- 6.Ali MA, et al. In: Fate of Arsenic in the Environment. Ahmed MF, editor. Dhaka, Bangladesh: Intl Training Network; 2003. pp. 7–20. [Google Scholar]
- 7.Meharg AA. Arsenic in rice—understanding a new disaster for South-East Asia. Trends Plants Sci. 2004;9:415–417. doi: 10.1016/j.tplants.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Williams PN, et al. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol. 2007;41:6854–6859. doi: 10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- 9.Schoof RA, et al. A market basket survey of inorganic arsenic in food. Food Chem Toxicol. 1999;37:839–846. doi: 10.1016/s0278-6915(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 10.Williams PN, Raab A, Feldmann J, Meharg AA. Market basket survey shows elevated levels of as in South Central US processed rice compared to California: Consequences for human dietary exposure. Environ Sci Technol. 2007;41:2178–2183. doi: 10.1021/es061489k. [DOI] [PubMed] [Google Scholar]
- 11.Williams PN, et al. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol. 2005;39:5531–5540. doi: 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- 12.Meharg AA, Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002;154:29–43. [Google Scholar]
- 13.Tripathi RD, et al. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2007;25:158–165. doi: 10.1016/j.tibtech.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi Y, et al. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol. 2004;38:1038–1044. doi: 10.1021/es034383n. [DOI] [PubMed] [Google Scholar]
- 15.Wysocki R, et al. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol Microbiol. 2001;40:1391–1401. doi: 10.1046/j.1365-2958.2001.02485.x. [DOI] [PubMed] [Google Scholar]
- 16.Meng YL, Liu ZJ, Rosen BP. As(III) and Sb(III) uptake by G1pF and efflux by ArsB in Escherichia coli. J Biol Chem. 2004;279:18334–18341. doi: 10.1074/jbc.M400037200. [DOI] [PubMed] [Google Scholar]
- 17.Bienert GP, Schuessler MD, Jahn TP. Metalloids: Essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem Sci. 2008;33:20–26. doi: 10.1016/j.tibs.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Meharg AA, Jardine L. Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol. 2003;157:39–44. doi: 10.1046/j.1469-8137.2003.00655.x. [DOI] [PubMed] [Google Scholar]
- 19.Chaumont F, Moshelion M, Daniels MJ. Regulation of plant aquaporin activity. Biol Cell. 2005;97:749–764. doi: 10.1042/BC20040133. [DOI] [PubMed] [Google Scholar]
- 20.Ma JF, et al. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- 21.Ma JF, Tamai K, Ichii M, Wu GF. A rice mutant defective in Si uptake. Plant Physiol. 2002;130:2111–2117. doi: 10.1104/pp.010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest KL, Bhave M. Major intrinsic proteins (MIPs) in plants: A complex gene family with major impacts on plant phenotype. Funct Integr Genomics. 2007;7:263–289. doi: 10.1007/s10142-007-0049-4. [DOI] [PubMed] [Google Scholar]
- 23.Dean RM, Rivers RL, Zeidel ML, Roberts DM. Purification and functional reconstitution of soybean nodulin 26. An aquaporin with water and glycerol transport properties. Biochemistry. 1999;38:347–353. doi: 10.1021/bi982110c. [DOI] [PubMed] [Google Scholar]
- 24.Choi WG, Roberts DM. Arabidopsis NIP2;1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. J Biol Chem. 2007;282:24209–24218. doi: 10.1074/jbc.M700982200. [DOI] [PubMed] [Google Scholar]
- 25.Wallace IS, Roberts DM. Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry. 2005;44:16826–16834. doi: 10.1021/bi0511888. [DOI] [PubMed] [Google Scholar]
- 26.Takano J, et al. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell. 2006;18:1498–1509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace IS, Roberts DM. Homology modeling of representative subfamilies of Arabidopsis major intrinsic proteins. Classification based on the aromatic/arginine selectivity filter. Plant Physiol. 2004;135:1059–1068. doi: 10.1104/pp.103.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red-cell Chip28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 29.Mitani N, Yamaji N, Ma JF. Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch. 2008;456:679–686. doi: 10.1007/s00424-007-0408-y. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005;46:1568–1577. doi: 10.1093/pcp/pci172. [DOI] [PubMed] [Google Scholar]
- 31.Xu XY, McGrath SP, Zhao FJ. Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol. 2007;176:590–599. doi: 10.1111/j.1469-8137.2007.02195.x. [DOI] [PubMed] [Google Scholar]
- 32.Ma JF, et al. An efflux transporter of silicon in rice. Nature. 2007;448:209–212. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- 33.Rosen BP. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp Biochem Physiol A. 2002;133:689–693. doi: 10.1016/s1095-6433(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 34.Silver S, Phung LT. A bacterial view of the periodic table: Genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol. 2005;32:587–605. doi: 10.1007/s10295-005-0019-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.