Abstract
Plant-made vaccines have been the subject of intense interest because they can be produced economically in large scale without the use of animal-derived components. Plant-made therapeutic vaccines against challenging chronic diseases, such as cancer, have received little research attention, and no previous human clinical trials have been conducted in this vaccine category. We document the feasibility of using a plant viral expression system to produce personalized (patient-specific) recombinant idiotype vaccines against follicular B cell lymphoma and the results of administering these vaccines to lymphoma patients in a phase I safety and immunogenicity clinical trial. The system allowed rapid production and recovery of idiotypic single-chain antibodies (scFv) derived from each patient's tumor and immunization of patients with their own individual therapeutic antigen. Both low and high doses of vaccines, administered alone or co-administered with the adjuvant GM-CSF, were well tolerated with no serious adverse events. A majority (>70%) of the patients developed cellular or humoral immune responses, and 47% of the patients developed antigen-specific responses. Because 15 of 16 vaccines were glycosylated in plants, this study also shows that variation in patterns of antigen glycosylation do not impair the immunogenicity or affect the safety of the vaccines. Collectively, these findings support the conclusion that plant-produced idiotype vaccines are feasible to produce, safe to administer, and a viable option for idiotype-specific immune therapy in follicular lymphoma patients.
Keywords: phase I clinical trial, plant-made pharmaceutical, single-chain antibodies
In recent years, non-Hodgkin's lymphoma (NHL) has become the most common hematologic malignancy in the United States with an estimated 54,000 new cases each year. Approximately 30% of these cases are follicular B cell lymphoma (1), with a median survival of 8–10 years from diagnosis (2). Most patients subjected to standard treatments, such as chemotherapy, radiation, or antibodies (2–9), still relapse (10–12). Newer approaches have focused on active immunotherapy through vaccination. For B cell lymphoma, the cell-surface Ig (Ig), or idiotype, is the unique tumor-specific antigen. In contrast to passive therapy, an idiotype-induced immune response is (i) highly tumor specific, thus sparing normal cells; (ii) potentially more durable; and (iii) protective against tumor cell variants that might “escape” under selective pressure, because the response is polyclonal (13–15).
There exists a 20-year history of idiotype vaccination for follicular lymphoma in animal models and clinical trials. The original vaccine manufacturing process, still used today in the majority of clinical research, used a patient's lymphoma B cells to derive a mouse/human heteromyeloma cell line for production of the tumor's monoclonal idiotype (16). Once purified, the idiotype antibody was chemically coupled to a highly immunogenic carrier protein, keyhole limpet haemocyanin (KLH), and administered with powerful immune modulatory agents, such as granulocyte–macrophage colony stimulating factor (GM-CSF) (17–19). The early studies showed that the administration of idiotype vaccines was safe and resulted in antitumor immune responses (20, 21). These responses were associated with more durable remissions and survival compared to historical controls (21, 22).
Because heteromyeloma cell lines are difficult and time-consuming to produce, more recent approaches used cloning of the tumor-derived cDNA and expression of the rearranged variable regions of the tumor idiotype (13, 23, 24). These idiotype fragments are typically cloned into a vector containing the Ig constant regions, and the resulting mAb is expressed in a mammalian or insect cell-based system. These animal cell culture-based recombinant Ig vaccines are under evaluation in phase III trials. Limitations of this approach still include cell culture expression of complex Ig proteins, expensive and lengthy culturing methods, and the need for KLH conjugation for successful immunity.
To simplify the production of idiotype vaccines, we expressed the single-chain form of the idiotype (scFv), which includes only the VH/VL domains and no constant regions, in a polypeptide of ≈30 kDa. In previous studies, scFv vaccines composed of either protein or DNA were shown to elicit anti-idiotype responses in animals (23, 25, 26) and were effective in blocking tumor progression in mouse models of lymphoma. We created a plant virus-based expression vector that could rapidly direct plants to produce high quantities of properly folded scFv corresponding to the 38C13 murine lymphoma model (27). Vaccination of mice with the 38C13 scFv purified from plants induced protective immunity against lymphoma challenge in the absence of KLH or other immune enhancing agents. Of the scFv vaccines derived from human lymphomas tested to date, >86% have been effectively manufactured by using this plant production system (28), which is comparable to standard hybridoma methods (29). Nonhybridoma methods, such as those reported in Redfern et al. (30), have claimed production rates of 100% (40/40). Such rates may be exceptions, because other rates are more comparable to our findings. These data demonstrated that the plant-based expression system is a robust production platform for generating idiotypic vaccines and that the resulting scFvs induced immune responses in a relevant mouse model. Furthermore, our transient plant-virus expression system could generate vaccines in less time than required with some animal cell-based approaches (31).
To establish the safety and immunogenicity of plant-produced scFv idiotype vaccines in patients, tumor-derived variable regions were cloned from patients and expressed as scFvs in plants. Chemotherapy-treated follicular lymphoma patients were vaccinated s.c. with purified scFv antigen at either 0.2 or 2.0 mg per dose, with or without 250 μg of the adjuvant GM-CSF administered s.c. next to the immunization site. The low dose of vaccine (0.2 mg) was selected because it represented the molar-equivalent idiotype dosage present in predecessor Ig-KLH idiotype vaccines; for determination of safety, a 10-fold higher dose (2.0 mg) was also included in the study. Thus the aim of this study was to rapidly produce well characterized, patient-specific lymphoma vaccines in plants and demonstrate their safety and immunogenicity.
Results
Patients.
The study was approved by the Institutional Review Board of the Stanford University Medical Center and was conducted under Food and Drug Administration IND 9283, sponsored by Large Scale Biology Corporation. All patients provided written informed consent. Of 27 patients who provided tissue for vaccine production, 23 were enrolled, and 16 patients completed the trial. Seven patients were excluded because of disease progression (three cases) or insufficient vaccine production (four cases). All 16 patients (Table 1) in this study had follicular B cell lymphoma, grade I or II based on the REAL/WHO classification (6, 32), and had received no prior therapy for lymphoma. Fourteen of the 16 subjects were Ann Arbor Stage IV, with the remainder being Stage III. All pathology was reviewed at Stanford University Medical Center. All patients required treatment for their lymphoma, as judged by the physician. Of the 16 subjects enrolled, 9 were female and 7 were male, with a mean age of 50 years (range 30–64). Fifteen subjects were Caucasian and one was Hispanic.
Table 1.
Patient characteristics
| Patient number | Gender | Age | Stage | Chemotherapy treatment | Chemotherapy response* | Bone marrow† | LDH | FLIPI |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 44 | IVA | CVPx8 | CR/CR | +/−/− | − | 2 |
| 2 | M | 45 | IIIA | CVPx6/CHOPx2 | PR/PR | −/−/− | + | 2 |
| 3 | F | 59 | IVA | CVPx6 | CR/PD | +/−/ND | − | 2 |
| 4 | M | 49 | IVA | CVPx6 | CRu/PD | +/−/− | + | 4 |
| 5 | F | 60 | IVA | CVPx8/CHOPx2 | PR/PR | +/+/ND | − | 2 |
| 6 | F | 45 | IVB | CVPx8 | CR/CR | +/−/− | + | 4 |
| 7 | F | 52 | IVB | CVPx8 | CRu/CRu | +/−/− | − | 2 |
| 8 | M | 56 | IVA | CVPx6 | CRu/CRu | +/−/− | − | 2 |
| 9 | F | 59 | IVA | CVPx6 | CRu/CRu | +/−/− | − | 1 |
| 10 | M | 58 | IVA | CVPx6 | CRu/CRu | +/−/ND | − | 2 |
| 11 | F | 61 | IVA | CVPx8 | CR/PR | +/+/− | + | 2 |
| 12 | F | 58 | IVA | CVPx8 | CRu/CRu | +/−/− | − | 2 |
| 13 | M | 46 | IVA | CVPx8/CHOPx2 | PR/PR | +/+/− | ND | 23 |
| 14 | M | 33 | IVA | CVPx6/CHOPx2 | CRu/CRu | +/−/− | − | 2 |
| 15 | F | 58 | IVA | CVPx8 | CRu/CRu | +/−/− | − | 1 |
| 16 | F | 67 | IIIA | CVPx7 | CRu/PD | −/−/ND | ND | 23 |
LDH, lactate dehydrogenase; FLIPI, Follicular Lymphoma International Prognostic Index; PR, partial remission; CR, complete remission; CRu, complete remission unconfirmed, PD, progressive disease.
*Chemotherapy response status after chemotherapy prior to vaccination.
†Bone marrow status indicates the presence of tumor cells in bone marrow at staging/pre-vaccine/post-vaccine; other variables shown are from time of staging prior to therapy.
Vaccine Production.
Cloning of the lymphoma tumor-specific heavy and light chain genes and the scFv into a tobacco mosaic virus (TMV) transient expression vector was successful for 100% of patients attempted (27 of 27 cases). Twenty-three of the 27 scFv vaccines (85%) were expressed and purified at sufficient yields for the clinical protocol requiring either high (2.0 mg) or low (0.2 mg) dosages. All 16 vaccines were released by quality assurance after meeting the product specifications established by FDA current good manufacturing practice (CGMP) guidelines [supporting information (SI) Table S1]. All vaccines were manufactured within the 6-month window after each patient completed their chemotherapy.
Of the 16 vaccines produced, 15 contained at least one N-linked glycan structure at putative sites coded in the heavy chain variable region as determined by MALDI-TOF mass spectral analysis (Table 2). Of the 15 glycosylated scFv proteins, 7 had fully occupied one available site (patients 2, 3, 14, and 15) or two sites (patients 1, 5, and 11) predicted by sequence analysis. In the remaining 8 of 15 glycoprotein vaccines, mixtures of species comprised of zero and one (patient 9), one and two (patients 4, 7, 8, 10, and 12), or two and three (patients 6 and 13) sites of glycosylation were observed (33). The variable region genes from patient 16 did not encode a glycosylation site and therefore that polypeptide was not glycosylated. Differences in the sizes of the scFv proteins and the number of stained band, due primarily to differences in glycosylation, could be seen by SDS/PAGE separation (Fig. S1).
Table 2.
Determination of vaccine glycan occupancy using MALDI-TOF mass spectrometry
| Patient number | Calculated sequence mass1, Da | MALDI-TOF mass, Da | Potential glycosylation sites | No. of occupied sites indicated | Average glycan molecular mass, Da |
|---|---|---|---|---|---|
| 1 | 25,869 | 28,252 | 2 | 2 | 1,192 |
| 2 | 25,836 | 27,223 | 1 | 1 | 1,387 |
| 3 | 24,582 | 25,824 | 1 | 1 | 1,242 |
| 4 | 25,678 | 27,571 and 28,778 | 2 | 1 and 2 | 1,721 |
| 5 | 26,646 | 29,414 | 2 | 2 | 1,384 |
| 6 | 25,651 | 28,006 and 29,189 | 3 | 2 and 3 | 1,179 |
| 7 | 26,146 | 28,760 | 2 | 1 and 2 | 1,307 |
| 8 | 25,668 | 26,840 and 28,060 | 2 | 1 and 2 | 1,184 |
| 9 | 24,945 | 24,939 and 26160 | 1 | 0 and 1 | 1,215 |
| 10 | 26,609 | 27,988 and 29,536 | 4 | 1 and 2 | 1,421 |
| 11 | 25,061 | 27,625 | 2 | 2 | 1,282 |
| 12 | 25,960 | 28,883 andt27,345 | 2 | 1 and 2 | 1,423 |
| 13 | 25,661 | 28,128 and 29,805 | 3 | 2 and 3 | 1,308 |
| 14 | 26,703 | 27,925 | 1 | 1 | 1,222 |
| 15 | 24,812 | 26,032 | 1 | 1 | 1,220 |
| 16 | 26,491 | 26,490 | 0 | 0 | N/A |
Study Design.
This phase I study was designed to evaluate the safety and immunogenicity of two dose levels of the study vaccine with and without GM-CSF. Vaccination was started ≈6 months after the last cycle of chemotherapy and given monthly for a total of six doses by s.c. injection (0.5 ml). As indicated, GM-CSF was given as an adjuvant in a site adjacent to the vaccine injection site on the day of the vaccination and for three consecutive days following. Patients were evaluated in clinic at days 1, 2, and 3 after each vaccination and by telephone on day 7. Long-term follow-up visits were scheduled at Months 9, 12, 18, 24, 30, and 36.
Fourteen of 16 patients were vaccinated within the planned 6- to 10-month time period postchemotherapy as specified by the clinical protocol. Patient 4 was vaccinated 11 months after chemotherapy due to a biopsy performed to confirm tumor progression in the mediastinum. Patient 16 was vaccinated 12 months after chemotherapy due to the need to integrate a metal affinity purification step for vaccine production. Three patients (3, 4, and 16) had minor progressive disease that did not require additional chemotherapy before starting vaccination and were allowed to continue treatment as protocol exemptions. Fifteen of the 16 patients received all six vaccinations, and patient 5 received four vaccinations before withdrawing due to disease progression. All 16 patients were evaluated for safety, immune responses, and clinical outcome. Seven of eight subjects in the GM-CSF group received all of their doses. Patient 15 received GM-CSF with only dose 1 due to development of a migraine headache with the initial injection and was subsequently switched to the high-dose group (no GM-CSF) to complete the study.
Safety.
The 16 individualized scFv vaccines were well tolerated by all patients. There were no serious adverse events observed during the study, and no patient withdrew from the study due to an adverse event caused by the vaccine (Table 3). One patient (15) discontinued receiving GM-CSF as described but successfully received all six vaccinations in the high-dose group without further event. Thirteen of 16 patients remain alive with a median follow-up of 78 months.
Table 3.
Number of adverse events within 7 days of each scFv vaccination
| Adverse events by body system | Group A (0.2 mg) | Group B (2.0 mg) | Group C (0.2 mg/GM) | Group D (2.0 mg/GM) |
|---|---|---|---|---|
| Gastrointestinal | ||||
| Swollen saliva glands | 0 | 0 | 0 | 1 |
| Nausea | 0 | 0 | 0 | 1 |
| General and administration site | ||||
| Redness at injection site | 0 | 1 | 16 | 17 |
| Fatigue | 1 | 1 | 0 | 2 |
| Bruising at injection site | 0 | 1 | 0 | 0 |
| Thigh pain | 0 | 1 | 1 | 0 |
| Swelling of thigh where injected | 0 | 0 | 16 | 13 |
| Immune system | ||||
| Allergies | 0 | 0 | 1 | 0 |
| Musculoskeletal and connective tissue | ||||
| Muscle aches | 0 | 0 | 0 | 1 |
| Leg cramps in the middle of the night | 0 | 0 | 0 | 1 |
| Nervous system | ||||
| Migraine-like headache with vision changes | 0 | 0 | 0 | 1 |
| Insomnia | 0 | 0 | 1 | 0 |
| Skin and subcutaneous tissue | ||||
| Rash | 0 | 1 | 0 | 0 |
Vaccine groups that also received GM-CSF are designated /GM.
Patients in groups A and B, who received scFv vaccine without GM-CSF, tolerated the vaccine with minimal local or systemic adverse events. Two subjects had temporary, mild injection-site reactions, and several subjects reported flu-like symptoms during the week after vaccination. These complaints were graded mild to moderate in severity and did not interfere with daily activities.
All of the patients who received GM-CSF with the vaccination reported reactions at the injection sites and mild to moderate flu-like symptoms. Some local reactions were graded as severe in intensity; however, all were resolved within a few days of vaccination and were consistent with the safety profile of GM-CSF at this dose and route of administration. One patient (11) developed hives and wheezing more than 7 days after receiving dose 6 with GM-CSF, but these symptoms resolved within 1 day and were not attributed to the vaccine due to the timing of onset.
Immune Responses.
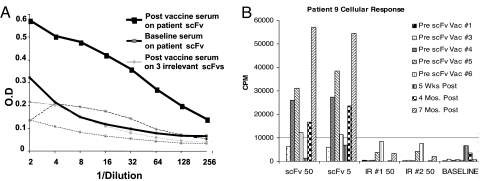
Blood was collected from each patient before the first vaccination and 28 days following each of the six vaccinations. Humoral immune responses were evaluated by using patient sera in an scFv antigen capture ELISA, and cellular responses were evaluated by in vitro proliferation of peripheral blood lymphocytes (PBL) to the scFv antigen compared to prevaccine samples. Responses that were 2-fold higher than background were considered positive (7 of 16 or 44%), and responses that were 2-fold higher than two different irrelevant scFvs were considered antigen specific (3 of 16 or 19%). A summary of immune responses is presented in Table 4. A representative example of a specific humoral response after the third immunization is shown in Fig. 1A. Here, the serial dilutions of the immune sera titered against the relevant idiotype scFv exhibit 2-fold higher OD values as compared to irrelevant idiotype scFv. Because we did not have rescue hybridoma protein for the majority of these patients, we were not able to address the relevance of these reactions to the actual tumor idiotype.
Table 4.
Patient vaccine groups and summary of immune responses
| Group | Vaccine/adjuvant | Patient number | Prevaccine status | Humoral response | Cellular response | Overall response | Timing of response |
|---|---|---|---|---|---|---|---|
| A | 0.2 mg | 1 | CR | — | + | + | 4 |
| 2 | PR | — | + | + | 2 | ||
| 3 | PD | +* | +* | +* | 2 | ||
| 4 | PD | — | +* | +* | 2 | ||
| B | 2.0 mg | 5† | PR | — | — | ND | — |
| 6 | CR(u) | — | — | — | — | ||
| 7 | CR | — | — | — | — | ||
| 8 | CR(u) | — | — | — | — | ||
| C | 0.2 mg with GM-CSF | 9 | CR(u) | +* | +* | +* | 1 |
| 10 | CR(u) | + | + | + | 1 | ||
| 11 | PR | + | +* | +* | 2 | ||
| 16 | PD | — | + | + | 3 | ||
| D | 2.0 mg with GM-CSF | 12 | CR(u) | + | +* | +* | 1 |
| 13 | PR | + | + | + | 1 | ||
| 14 | CR(u) | +* | +* | +* | 1 | ||
| 15‡ | CR(u) | — | — | — | — |
*Specific responses.
†Patient 5 received only four vaccine doses before discontinuing treatment due to disease progression (see text).
‡Patient 15 received only one of six planned GM-CSF doses; five doses of 2.0 mg vaccine were administered without GM-CSF.
Fig. 1.
Examples of specific humoral and cellular immune responses after scFv vaccination. (A) Patient sera were analyzed for specific anti-idiotype responses by ELISA at baseline (preimmunization) and after the third immunization against the idiotype-relevant scFv. Three irrelevant scFv targets were also used to establish specificity of the response, as indicated. Sera were diluted 2-fold, and reactivity was determined by anti-human IgG-HRP followed by ABTS development. OD was measured by using a SpectraMax plate reader, and data were plotted by using Excel. (B) Positive, sustained cellular proliferation response after vaccination. Patient 9 received low-dose scFv vaccine with GM-CSF. After the PBMCs were stimulated with relevant (scFv) at 0.5, 5.0, or 50 μg/ml or irrelevant (Ir1 or Ir2) scFv at 50 μg/ml. A specific positive cellular response was measured prevaccine 3 that was sustained to 7 months postimmunization.
Cellular responses (T cell proliferation) were measured in 11 of 16 patients (69%), of which 6 (38%) were specific to the patient scFv. Fig. 1B illustrates a specific cellular response starting after the second immunization (prevaccine 3) and sustained at high levels up to 7 months after vaccine. To our knowledge, such sustained, high-level cellular proliferative responses have not been observed in previous vaccine trials.
Of the 11 patients who had cellular responses, seven of these patients also had a humoral response (34), and most cellular (7 of 11) and humoral (6 of 7) responders received the vaccine in conjunction with GM-CSF, suggesting an important role for GM-CSF in this system. Conversely, all patients who generated a humoral response also generated a cellular response (Table 4). In groups that received vaccine with GM-CSF, five of seven patients (71%) had a specific cellular response and two of seven (29%) showed a specific humoral response. Although the overall immune response rate was 69%, only 44% of patients met antigen-specific response criteria, based on a lack of reactivity with irrelevant scFvs.
Clinical Outcomes.
Thirteen of 16 patients were in remission at the time of vaccination, and three were vaccinated with minor progressive disease not requiring treatment. Currently, 13 of the 16 patients remain alive with a postchemotherapy follow-up of 24–89 months (median of 78 months). One patient (patient 7) from the original cohort remains in remission since the original treatment. Patients 2,#10, and 13 died at 52, 47, and 24 months, respectively, after completion of chemotherapy. Patients 2 and 13 had disease at the time of death; patient 10 died due to graft-versus-host disease after a subsequent bone marrow transplant. The median time to progression was 23.5 months, with no obvious differences between patients with or without an immune response. All other patients progressed at varying times and went on to receive additional treatment. Patient 9, who had a sustained specific cellular immune response, exhibited disease regression while on observation and maintained this complete remission for 3 years after the final vaccine.
Discussion
This study reports the parenteral administration of a plant-produced vaccine in humans. Plant-produced scFv idiotypic glycoprotein vaccines were well tolerated, safe, and immunogenic in a majority of patients. Only minor adverse events, primarily at the site of injection, were observed in this study, and these reactions were attributed to the coadministration of the GM-CSF adjuvant. Although the group sizes in the study do not allow statistical evaluation, the high percentage of responses observed in the GM-CSF groups suggests that the use of adjuvant is important. The augmenting effect of GM-CSF is in agreement with data from clinical investigations on the immunogenicity of idiotypic mAbs conjugated to KLH (35).
Although the scFv vaccine design comprises a relatively small protein that is only a fraction of the size of a full-size Ig molecule, the idiotype scFv was shown to induce an immune response in a high percentage of the patients (11 of 15 or 73%). Moreover, over half (6 of 11) of the responders were characterized as having a specific immune response, as measured by reactivity with relevant scFv idiotype but not irrelevant scFv. Interestingly, no responses were obtained in the high-dose group (2.0 mg per dose) in the absence of the adjuvant GM-CSF, whereas the four low-dose patients all achieved immune responses without GM-CSF, a likely consequence of the small sample size.
These are the first immune responses observed against a s.c.-administered idiotype vaccine in the absence of a highly immunogenic carrier protein, such as KLH. Our data indicate that 3 of 16 patients mounted a vaccine-specific humoral immune response. Although this number is low, it is comparable to a cohort in another study reported by Timmerman (36) in which hybridoma-produced Ig was introduced into dendritic cells before vaccination without KLH conjugation. In that study, only 1 of 12 patients had an anti-Id specific humoral response. Both our study and the Timmerman study suggest that KLH may be an important vaccine component to stimulate anti-idiotype- or anti-vaccine-specific antibody responses.
KLH carrier protein was not used in this study in order to establish the safety of the plant-produced products in patients in the absence of such a conjugate and because tumor protective immune responses were observed without KLH in animal models of lymphoma (27). In human studies where KLH conjugation was used, overall humoral response rates were higher, such as the response rate of 41% (17 of 41) reported by Hsu (21) and 65% (13 of 20) reported by Inoges (29). However, these two studies used additional adjuvants, complicating direct comparisons. The remaining cohort in the Timmerman study also showed a higher humoral response rate of 46% (6 of 13) when KLH conjugation was used. Combined with GM-CSF adjuvant, scFv-KLH conjugates could also constitute more effective vaccines.
Interestingly, in contrast to the low number of humoral responses, the majority of patients mounted a cellular response, and most cellular immune responses were detected after only the first or second dose of vaccine (in 9 of 16 patients), with one response detected after the third dose (Table 4). Also, all three patients with progressive disease during this trial were able to mount an immune response. Previous studies rarely reported responses after a single vaccination or in patients with existing tumor burden. We have also observed continuing and durable immune responses in some patients (for example, patient 9; Fig. 1B), in contrast to previous vaccine trials. In fact, the scFv idiotypic subunit design, which lacks the highly conserved constant regions, may have been paramount in the rapid and sustained induction of immune responses, because the presence of conserved constant region sequences may present a higher threshold for obtaining antigen-specific immunity (37, 38).
Although it could be postulated that both cellular and humoral immune responses might have been generated against neoantigens created by linker-variable region fusions, this is unlikely for several reasons. First, nonspecific humoral responses were measured by reading above-background immune responses to nonvaccine scFv that have similar framework regions but nonidentical linker sequences, which argues against immunity to linker sequences. Second, the amino acids in this selected linker composition (STAG) are highly unfavorable for MHC class I or II epitope binding, whereas hydrophobic, charged, or cyclic amino acid anchor residues are favored for optimal MHC interaction (39, 40) although this cannot be absolutely ruled out.
It has also been hypothesized that the presence of plant-specific glycan structures in the vaccines could affect the speed and specificity of the immune responses. However, immune responses were detected in the one patient immunized with nonglycosylated scFv (patient 16; Tables 2 and 4) and in one (patient 10) of two patients (patients 5 and 10) that were immunized with vaccines that contained no plant-specific carbohydrate linkages but did contain the core mannosyl terminal structure (GlcNAc2-Man3) conserved between mammalian and plant glycans. Compositional analysis of the remaining 13 glycosylated vaccines (data not shown) found plant-specific glycans with the predominant structures GlcNAc2-Man3, GlcNAc2-Man3-GlcNAc1, and GlcNAc2-Man3-GlcNAc2. Each structure contained plant-specific carbohydrate linkages, α (1,3) fucose and β (1,2) xylose, attached to the first GlcNAc residue or the branching mannose residue, respectively. Nine patients receiving vaccines with plant-specific carbohydrate linkages mounted measurable immune responses, and six were deemed specific, by lack of reactivity to other irrelevant scFvs containing similar plant glycan content. The remaining four patients who received vaccines with plant-specific glycan structures showed no measurable immune responses. Taken together, these results strongly suggest that the immune responses were not directed toward plant-specific glycan structures but rather to the idiotype sequence itself and that the adjuvant effects of GM-CSF were the most relevant variable regarding whether a patient responded to the vaccine.
Several conclusions can be drawn from the study. First, there was no obvious advantage to a high dose (2.0 mg) of the vaccine versus the equivalent full-Ig molar dose of 0.2 mg. Secondly, the addition of GM-CSF as an adjuvant was safe and resulted in an apparent higher frequency of immune responses. All seven patients who received their planned dosing of GM-CSF mounted an immune response, whereas only four of eight in the non-GM-CSF arms mounted an immune response. In these patients, the immune response data showed no correlation with clinical outcome because there was no difference in median time to progression (23.5 months) between immune responders and nonresponders, comparable to the time to progression expected for follicular lymphoma patients after standard chemotherapy (2, 6). No comparative conclusions about vaccine efficacy can be made, and efficacy was not a priority or an endpoint in this study. Clearly, changes in vaccine formulation, such as conjugation to KLH, may significantly improve clinical responses to scFv vaccines either by extending duration of antigen presentation or by improving the immune stimulatory context of antigen presentation.
The transient plant-viral expression system used to produce the vaccines applied in these clinical studies can rapidly produce the needed amounts of idiotype vaccine. Under CGMP manufacturing, using only manual methods and without optimizing production efficiency, we were able to generate patient-specific scFv typically within 12–16 weeks of receiving biopsy specimens. Rapid manufacturing has a direct impact on the population of patients that could be served by these vaccines, including follicular NHL patients that are newly diagnosed and have not yet received conventional (immunosuppressive) chemotherapy and/or immunotherapy. The longer times required to produce personalized vaccines when using animal cell approaches restrict these vaccines to use in second-line or adjuvant therapy (41, 42). Thus, vaccines produced by transient expression in plants could reach a larger population of NHL patients.
We have documented the safety of plant-produced idiotypic vaccines in NHL patients and their ability to induce tumor-specific immune responses, without the universally used KLH carrier. The idiotype vaccines' subunit design, lacking constant region, may have contributed to improved immunity to self antigens, even in patient cohorts vaccinated without adjuvant. Thus, plant-based production could provide a safe and effective alternative vaccine formulation with new features over those previously described.
Materials and Methods
Patients.
The preferred chemotherapy regimen was Cytoxan 400 mg/m2 given orally once a day on days 1–5, Vincristine 1.4 mg/m2 on day 1 with a 2-mg maximum, and Prednisone 100 mg/m2 on days 1–5 (CVP). Patients who did not have a response after four to six cycles of CVP were changed to Cytoxan 750 mg/m2, Adriamycin 50 mg/m2, Vincristine as above on day 1, and Prednisone 50 mg/m2 on days 1–5 (CHOP). No patient received >10 cycles of chemotherapy. Patients received their chemotherapy either at Stanford or at the site of their primary oncologist. All responses were classified according to the Cheson criteria (32) as partial remission (PR), complete remission (CR), complete remission unconfirmed (CRu), or progressive disease (PD). Patients underwent a 6- to 10-month recovery period before vaccination and had to remain in remission; if progression occurred, it had to be judged not to require immediate treatment. Staging studies conducted before vaccination included CT scans of the chest, abdomen, and pelvis; bilateral bone marrow biopsies and complete blood cell count; lactate dehydrogenase; Follicular Lymphoma International Prognostic Index; and blood chemistries. Laboratory tests and physical exams were repeated at each clinic visit, and CT scans were performed at 1 month after the sixth vaccination and then every 6 months for 30 months, or as clinically indicated.
Vaccine Production, Release, and Glycan Analysis.
Production of scFv clones, plant inoculation, and scFv purification was carried out as previously described (28) and as shown in Fig. S1. All scFv vaccines were manufactured under FDA CGMP guidelines, as presented in the SI Text.
The quality of vaccine products was monitored by using CGMP quality control and quality assurance guidelines. Product release assays were used to assess product identity; tumor relevance, purity, and potency; and other product properties including safety (Table 1 and SI Text). The absence of infectious TMV vector was confirmed by local lesion assay using Nicotiana tabacum NN leaf inoculation methods. The quality of the vaccine products was also monitored during a 12- to 24-month stability study by using a subset of the product release tests used for clinical application (34).
Glycan analysis, presented in Table 2, was carried out as described in detail elsewhere (refs. 33 and 34; F.V., K.M.H., and E.L.W., unpublished data). Briefly, each patient's scFv sequence was analyzed for expected glycosylation sites based on the presence of an N-glycosylation sequence, Asn-X-Ser/Thr. Patient scFv protein was analyzed by MALDI-TOF mass analysis, and initially the number of glycosylation sites occupied were determined by the difference in mass from the expected amino acid weight (based on protein sequence and observed mass) divided by the average glycan mass of 1,220 Da. Glycosylation is also clearly observed in the differential migration of scFv proteins by SDS/PAGE analysis (Fig. S1). Additional analyses by tryptic digestion of scFv protein to generate peptide fragments predicted to contain N-liked glycosylation sites were further analyzed by MALDI-TOF. The presence of glycan occupation was confirmed, and the mass of each glycan was established (33). The exact composition of glycan identity will be presented elsewhere (43).
Vaccine Administration.
Vaccine was administered at 0.2 mg or at 2.0 mg per dose, according to the assigned patient group (Table 4). The 0.2-mg dose is the molar equivalent of the whole Ig idiotype protein dose used in previous clinical studies (21). Patients were divided into four cohorts of four patients each, with eight patients receiving the low dose and eight receiving the high dose. In each dose group, four patients also received GM-CSF. The vaccine was split into two 0.25-ml injections and given s.c. into each thigh, once a month for 6 months, with or without GM-CSF. GM-CSF was split into two s.c. injections administered at the vaccine site at a dose of 250 μg on the day of vaccination and for each of three consecutive days thereafter.
Immune Response Assays.
T cell proliferative responses were measured at baseline and monthly as described previously (21). Peripheral blood mononuclear cells (PBMCs) were cultured in replicates of four at 50,000 cells per well of 0.2 ml containing media alone, patient scFv idiotype at four different concentrations (0.05, 0.5, 5.0, and 50 μg/ml), or at least two irrelevant scFv proteins at three different concentrations (0.5, 5.0, and 50 μg/ml). Cells were cultured for 3 days in media alone. On day 4, cells were split 1:2 into medium containing 5% FBS and 30 units/ml IL-2. Cells were pulsed 2 days later with 1 μCi per well of [3H] and harvested, and thymidine incorporation was determined. A positive response was greater than twice background on two separate occasions. A specific response was greater than twice that of any irrelevant scFv tested. For patients exhibiting positive and specific responses, assays were repeated until negative. Antibody immune responses were measured by ELISA as previously described (27). Positive and specific response criteria were the same as that used for cellular responses. Statistics analyses were performed with the GraphPad Prism software program. Time to progression and overall survival were measured from the end of the last chemotherapy cycle.
Supplementary Material
Acknowledgments.
We thank the Large Scale Biology Corporation team for the production and validation of the patient-specific vaccines, the clinical staff at Stanford University Medical Center for the administration of the vaccines, and Dr. Yonnie Wu for his contribution to the glycan analyses.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803636105/DCSupplemental.
References
- 1.Greiner TC, Medeiros LJ, Jaffe ES. Non-Hodgkin's lymphoma. Cancer. 1995;75:370–380. doi: 10.1002/1097-0142(19950101)75:1+<370::aid-cncr2820751319>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Horning SJ. Natural history of and therapy for the indolent non-Hodgkin's lymphomas. Semin Oncol. 1993;20:75–88. [PubMed] [Google Scholar]
- 3.Hiddemann W, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (chop) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with chop alone: Results of a prospective randomized study of the German low-grade lymphoma study group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Ilizaliturri FJ, Gowda A, Czuczman MS. Development of targeted therapies for b-cell non-Hodgkin lymphoma and multiple myeloma. Clin Adv Hematol Oncol. 2004;2:606–618. [PubMed] [Google Scholar]
- 5.Rao AV, Akabani G, Rizzieri DA. Radioimmunotherapy for non-Hodgkin's lymphoma. Clin Med Res. 2005;3:157–165. doi: 10.3121/cmr.3.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage JO, Harris NL, Beirman P. Non-Hodgkin's Lymphomas. Philadelphia: Lippincott-Raven; 2001. [Google Scholar]
- 7.Maloney DG, et al. Idec-c2b8: Results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 8.Maloney DG, et al. Idec-c2b8 (rituximab) anti-cd20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 9.Grillo-Lopez AJ. Zevalin: The first radioimmunotherapy approved for the treatment of lymphoma. Exp Rev Anticancer Ther. 2002;2:485–493. doi: 10.1586/14737140.2.5.485. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at the University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2006;24:1582–1589. doi: 10.1200/JCO.2005.03.3696. [DOI] [PubMed] [Google Scholar]
- 11.Ganti AK, et al. Patients with grade 3 follicular lymphoma have prolonged relapse-free survival following anthracycline-based chemotherapy: The Nebraska lymphoma study group experience. Ann Oncol. 2006;17:920–927. doi: 10.1093/annonc/mdl039. [DOI] [PubMed] [Google Scholar]
- 12.Plancarte F, et al. Follicular lymphoma in early stages: High risk of relapse and usefulness of the follicular lymphoma international prognostic index to predict the outcome of patients. Eur J Haematol. 2006;76:58–63. doi: 10.1111/j.1600-0609.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 13.Timmerman JM, Levy R. The history of the development of vaccines for the treatment of lymphoma. Clin Lymph. 2000;1:129–140. doi: 10.3816/clm.2000.n.011. [DOI] [PubMed] [Google Scholar]
- 14.Press OW, Leonard JP, Coiffier B, Levy R, Timmerman J. Immunotherapy of non-Hodgkin's lymphomas. Hematology. 2001:221–240. doi: 10.1182/asheducation-2001.1.221. [DOI] [PubMed] [Google Scholar]
- 15.Coscia M, Kwak LW. Therapeutic idiotype vaccines in b lymphoproliferative diseases. Exp Opin Biol Ther. 2004;4:959–963. doi: 10.1517/14712598.4.6.959. [DOI] [PubMed] [Google Scholar]
- 16.Carroll WL, et al. Idiotype variant cell populations in patients with b cell lymphoma. J Exp Med. 1986;164:1566–1580. doi: 10.1084/jem.164.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak LW, et al. Induction of immune responses in patients with b-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 18.Tao MH, Levy R. Idiotype/granulocyte-macrophage colony-stimulating factor fusion protein as a vaccine for b-cell lymphoma. Nature. 1993;362:755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- 19.Hsu FJ, et al. Clinical trials of idiotype-specific vaccine in b-cell lymphomas. Ann NY Acad Sci. 1993;690:385–387. doi: 10.1111/j.1749-6632.1993.tb44039.x. [DOI] [PubMed] [Google Scholar]
- 20.Nelson EL, et al. Tumor-specific, cytotoxic t-lymphocyte response after idiotype vaccination for b-cell, non-Hodgkin's lymphoma. Blood. 1996;88:580–589. [PubMed] [Google Scholar]
- 21.Hsu FJ, et al. Tumor-specific idiotype vaccines in the treatment of patients with b- cell lymphoma—Long-term results of a clinical trial. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- 22.Hsu FJ, et al. Vaccination of patients with b-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins RE, et al. Idiotypic vaccination against human b-cell lymphoma. Rescue of variable region gene sequences from biopsy material for assembly as single-chain fv personal vaccines. Blood. 1994;83:3279–3288. [PubMed] [Google Scholar]
- 24.Ruffini PA, Di Nicola M, Carlo-Stella C, Siena S, Gianni AM. Genetic idiotypic and tumor cell-based vaccine strategies for indolent non Hodgkin's lymphoma. Curr Gene Ther. 2005;5:511–521. doi: 10.2174/156652305774329221. [DOI] [PubMed] [Google Scholar]
- 25.Hakim I, Levy S, Levy R. A nine-amino acid peptide from IL-1β augments antitumor immune responses induced by protein and DNA vaccines. J Immunol. 1996;157:5503–5511. [PubMed] [Google Scholar]
- 26.Spellerberg MB, et al. DNA vaccines against lymphoma: Promotion of anti-idiotypic antibody responses induced by single chain fv genes by fusion to tetanus toxin fragment c. J Immunol. 1997;159:1885–1892. [PubMed] [Google Scholar]
- 27.McCormick AA, et al. Rapid production of specific vaccines for lymphoma by expression of the tumor-derived single-chain fv epitopes in tobacco plants. Proc Natl Acad Sci USA. 1999;96:703–708. doi: 10.1073/pnas.96.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick AA, et al. Individualized human scfv vaccines produced in plants: Humoral anti-idiotype responses in vaccinated mice confirm relevance to the tumor ig. J Immunol Methods. 2003;278:95–104. doi: 10.1016/s0022-1759(03)00208-4. [DOI] [PubMed] [Google Scholar]
- 29.Inoges S, et al. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J Natl Cancer Inst. 2006;98:1292–1301. doi: 10.1093/jnci/djj358. [DOI] [PubMed] [Google Scholar]
- 30.Redfern CH, et al. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24:3107–3112. doi: 10.1200/JCO.2005.04.4289. [DOI] [PubMed] [Google Scholar]
- 31.Levy R, Dilley J. Rescue of immunoglobulin secretion from human neoplastic lymphoid cells by somatic cell hybridization. Proc Natl Acad Sci USA. 1978;75:2411–2415. doi: 10.1073/pnas.75.5.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheson BD, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI sponsored international working group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 33.White E L, et al. Proc Am Soc Mass Spec Conf Mass Spec Allied Topics. 2003;51:314. [Google Scholar]
- 34.Hanley KM, et al. Proc Am Soc Mass Spec Conf Mass Spec Allied Topics. 2003;51:150. [Google Scholar]
- 35.Bendandi M, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 36.Timmerman JM, et al. Idiotype-pulsed dendritic cell vaccination for b-cell lymphoma: Clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 37.Syrengelas AD, Levy R. DNA vaccination against the idiotype of a murine b cell lymphoma: Mechanism of tumor protection. J Immunol. 1999;162:4790–4795. [PubMed] [Google Scholar]
- 38.Timmerman JM, Caspar CB, Lambert SL, Syrengelas AD, Levy R. Idiotype-encoding recombinant adenoviruses provide protective immunity against murine b-cell lymphomas. Blood. 2001;97:1370–1377. doi: 10.1182/blood.v97.5.1370. [DOI] [PubMed] [Google Scholar]
- 39.Fagerberg T, Cerottini JC, Michielin O. Structural prediction of peptides bound to mhc class i. J Mol Biol. 2006;356:521–546. doi: 10.1016/j.jmb.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen M, et al. Improved prediction of mhc class i and class ii epitopes using a novel gibbs sampling approach. Bioinformatics. 2004;20:1388–1397. doi: 10.1093/bioinformatics/bth100. [DOI] [PubMed] [Google Scholar]
- 41.Timmerman J, Vose J, Levy R, Mayo M, Denney D. Long-term follow-up of patients treated in a phase 2 trial with MyVax personalized immunotherapy (recombinant Id-KLH with GM-CSF) after chemotherapy as initial treatment for follicular non-Hodgkin's lymphoma (NHL) Blood. 2005;106:2438. [Google Scholar]
- 42.Leonard JP, Mason K, Theriault T, Milner K, Sornasse T, et al. Preliminary immune response (IR) results of a phase 2 study with idiotype (Id) immunotherapy after treatment with CVP and rituximab for follicular non-Hodgkin's lymphoma (FL) J Clin Oncol. 2006;24:7529. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.