Abstract
Through a mechanism that is unclear, systemic fluoroquinolones and tetracyclines can attain higher levels in gingival fluid than in blood. We hypothesized that gingival fibroblasts take up and accumulate these agents, thereby enhancing their redistribution to the gingiva. Using fluorescence to monitor transport activity, accumulation of fluoroquinolones and tetracyclines was characterized in cultured human gingival fibroblast monolayers. Both were transported in a concentrative, temperature-dependent and saturable manner. Fibroblasts transported ciprofloxacin and minocycline with Km values of 200 and 108 μg/ml, respectively, at maximum velocities of 4.62 and 14.2 ng/min/μg cell protein, respectively. For both agents, transport was most efficient at pH 7.2 and less efficient at pH 6.2 and 8.2. At steady state, the cellular/extracellular concentration ratio was >8 for ciprofloxacin and >60 for minocycline. Thus, gingival fibroblasts possess active transporters that could potentially contribute to the relatively high levels these agents attain in gingival fluid.
Keywords: fluoroquinolone, tetracycline, antimicrobial chemotherapy, periodontitis
INTRODUCTION
Scaling and root planing disperse bacterial plaque and arrest the progression of periodontitis in many individuals. However, refractory and aggressive forms of periodontitis may be resistant to this treatment because the associated pathogens (Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis) are capable of invading epithelial cells lining the pocket or gingival crevice (Christersson et al., 1987; Fives-Taylor et al., 1996; Lamont et al, 1995). In this manner, invasive pathogens can evade the host response and gain access to nutrients inside the host. Antimicrobial agents are frequently used as adjuncts to help eliminate these bacteria. Fluoroquinolones and tetracyclines are particularly useful because they can penetrate epithelial cells. Fluoroquinolones are highly efficient in killing A. actinomycetemcomitans, while tetracyclines are capable of inhibiting both A. actinomycetemcomitans and P. gingivalis (Pavicic et al., 1992; van Winkelhoff et al., 1996). Interestingly, both classes of antibiotics appear to preferentially distribute in the gingival fluid (GF). When taken by the systemic route, tetracyclines attain GF levels that are approximately 4–5 fold higher than serum levels, while ciprofloxacin reaches GF levels that are about 4.5 fold higher (Pascale et al., 1986; Ciancio et al. 1980; Conway et al., 2000).
Little is known about the mechanisms by which these agents concentrate in GF. Since polymorphonuclear leukocytes (PMNs) take up and accumulate ciprofloxacin and other fluoroquinolones (Easmon and Crane, 1985; Perea et al., 1992; Garraffo et al., 1991), it has been suggested that they might carry ciprofloxacin with them as they migrate to inflamed periodontal sites. In support of this hypothesis, recent studies demonstrated that root planing results in a small (10%), but statistically significant, decrease in GF ciprofloxacin levels at sites that initially exhibit inflammation. However, GF ciprofloxacin levels are still several-fold higher than serum levels even in subjects with healthy gingiva (Conway et al., 2000). This suggests that inflammation is not the major determinant of fluoroquinolone accumulation in GF. GF originates from the vessels of the gingival plexus, seeps through the connective tissue and passes through the junctional epithelium (Schroeder and Listgarten, 1997). Since the junctional epithelium is a relatively leaky epithelial barrier (Schroeder, 1981), it probably does not play a role in concentrating antimicrobial agents in the GF. We hypothesize that fibroblasts in the gingival connective tissue accumulate fluoroquinolones and tetracyclines, thereby enhancing their redistribution to gingiva and increasing their levels in GF. Our results support this hypothesis and provide a better understanding of the distribution of these agents in the gingiva.
MATERIALS AND METHODS
Isolation and culture of gingival fibroblasts
Fibroblasts were isolated from explants obtained from healthy adult interproximal papillae as previously described (Mariotti and Cochran, 1990), using a protocol and informed consent procedure approved by the Institutional Review Board. Cells were cultured at 37°C in 5% CO2 in minimal essential medium (MEM, Life Technologies Inc, Rockville, MD) supplemented with 2 mM glutamine and 10% heat-inactivated fetal bovine serum. For the experiments described below, fibroblasts were seeded into 24-well cell culture plates and fed every 3 days until they formed a confluent monolayer. Cells were used between passage numbers 4 to 15.
Assay of fluoroquinolone and tetracycline transport
Transport was assayed by measuring cell-associated fluoroquinolone or tetracycline fluorescence. Multiwell culture plates containing confluent cell monolayers were washed four times with Hanks balanced salts solution (HBSS), overlaid with 0.2 ml/well HBSS, and warmed to 37°C prior to assay. In the ciprofloxacin transport assays, 0.2 ml of warm HBSS containing twice the desired final fluoroquinolone concentration was simultaneously added to each well with multichannel pipettes. After incubation at 37°C for the indicated times, the fluoroquinolone solutions were quickly removed. Each well was rapidly washed four times with HBSS to eliminate extracellular antibiotics, using the cluster-tray method described by Gazzola et al. (1981). Cell monolayers were lysed in 1 ml of 100 mM glycine (pH 3.0) with a scraper. The lysate was centrifuged at 13,000 × g for 6 minutes and its fluorescence was measured as previously described (Walters et al., 1999). Transport of tetracyclines was assayed using a similar approach, except that the cells were lysed in 1 ml of water. For minocycline, the lysate was added to 1 ml of ethylene glycol containing 200 mM citric acid and 200 mM magnesium acetate prior to measurement of the fluorescence (Lever, 1972). Calibration plots were constructed to relate fluorescence to cell antibiotic content, which was normalized to cell protein using the method of Bradford (1976).
To determine the affinity and velocity of transport, the kinetics of transport were measured during the linear initial phase (0 to 3 minutes) and analyzed by the Lineweaver-Burk method. EnzPack for Windows (Biosoft, Ferguson, MO) was used to derive the Michaelis constant (Km) and maximum transport velocity (Vmax) values from regression lines obtained with the plotted data. Several organic cations inhibited transport and altered the Lineweaver-Burk plot intercepts. The pattern of alterations produced by these agents was used to determine the mechanism of inhibition and the inhibition constant (Ki).
Intracellular volume measurements
To calculate intracellular antibiotic concentrations, the intracellular content was divided by intracellular volume. The latter was measured by equilibrating fibroblast suspensions with [3H]-water (5 μCi/ml, NEN Life Science Products) (Garraffo et al., 1991). After incubation for 10 minutes, cells were rapidly pelleted through oil as previously described (Walters et al., 1999). The pellet was lysed and counted with a liquid scintillation system. To correct for the extracellular water trapped in the pellet, cells were equilibrated with [14C]-inulin (2 μCi/ml, NEN Life Science Products) and processed similarly.
RESULTS
Gingival fibroblasts accumulated ciprofloxacin and minocycline in a saturable and temperature-dependent manner (Figure 1). Uptake of both agents was strongly inhibited at 4° C. Their kinetics yielded linear plots when analyzed by the Lineweaver-Burk method. Ciprofloxacin was transported with a Km of 200 μg/ml at a Vmax of 4.62 ng/min/μg cell protein (Table 1). Ofloxacin and moxifloxacin were transported with similar affinity, but the Vmax value for moxifloxacin was higher than for ciprofloxacin. Minocycline was transported with a Km of 108 μg/ml and a Vmax of 14.2 ng/min/μg protein, while doxycycline and tetracycline were transported with a higher Km at a much lower Vmax. Despite the relatively low affinity of transport, fibroblasts accumulated high levels of ciprofloxacin and minocycline. Incubation with 10 μg/ml of either agent yielded steady-state intracellular concentrations of 82.8 ± 4.4 μg/ml for ciprofloxacin and 610 ± 2.6 μg/ml for minocycline (not shown).
Figure 1.
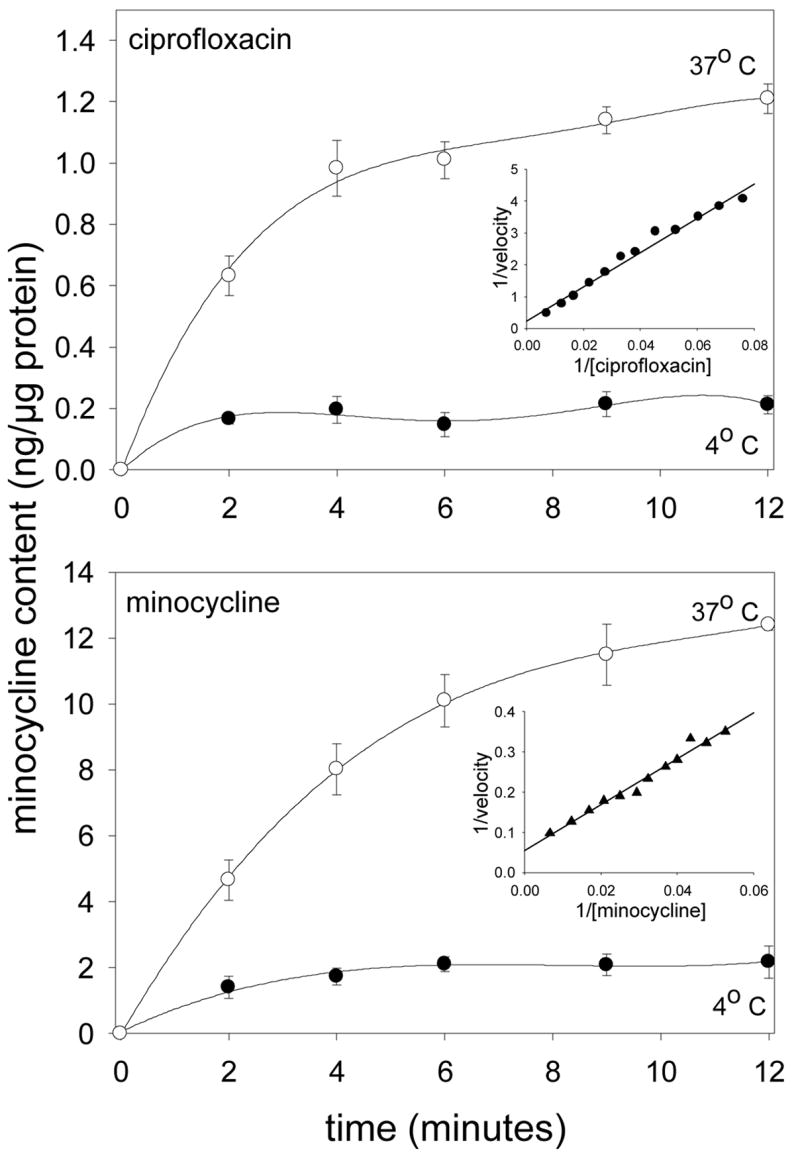
Temperature-dependence of ciprofloxacin and minocycline accumulation by gingival fibroblasts. After incubation of cell monolayers in HBSS at 4° (●) and 37° C (○), 20 μg/ml ciprofloxacin or minocycline was added and uptake was monitored over the indicated time intervals. The data represent the mean ± SEM of 4 experiments. Insets: Lineweaver-Burk plots of ciprofloxacin (•) and minocycline (▲) transport observed over 3 minutes at 37° C
Table 1.
Kinetic Constantsa for Transport of Antimicrobial Agents by Gingival Fibroblasts
| Agent | Km | Vmax | |
|---|---|---|---|
| μM | (μg/ml) | ng/min/μg protein | |
| Minocycline | 219 ± 14.0 | (108 ± 6.90) | 14.2 ± 0.77 |
| Doxycycline | 432 ± 29.3 | (208 ± 14.1) | 2.48 ± 0.22 |
| Tetracycline | 293 ± 23.9 | (141 ± 11.5) | 1.79 ± 0.18 |
| Ciprofloxacin | 628 ± 43.5 | (200 ± 14.4) | 4.62 ± 0.31 |
| Ofloxacin | 634 ± 56.8 | (229 ± 20.5) | 4.02 ± 0.21 |
| Moxifloxacin | 457 ± 37.9 | (200 ± 16.6) | 8.57 ± 0.67 |
Determined by Lineweaver-Burk analysis of transport activity assayed during the rapid initial phase of uptake (3 minutes). Results are expressed as mean ± SEM of at least 5 experiments
To determine the effect of pH on transport, the kinetics of uptake were analyzed between pH 6.2 and 8.2 (Figure 2, top). Changes in pH had a significant effect on the efficiency of transport (assessed by the Vmax/Km ratio; P < 0.05, ANOVA). Transport of both agents was most efficient at pH 7.2, but Vmax/Km decreased by nearly 35% at pH 6.2 (P < 0.05 Dunnett’s test for both) and 25% at pH 8.2 (P < 0.05 for ciprofloxacin). The transporters were further characterized by comparing transport activity in the presence and absence of sodium. Elimination of sodium failed to block the transport of ciprofloxacin or minocycline.
Figure 2.
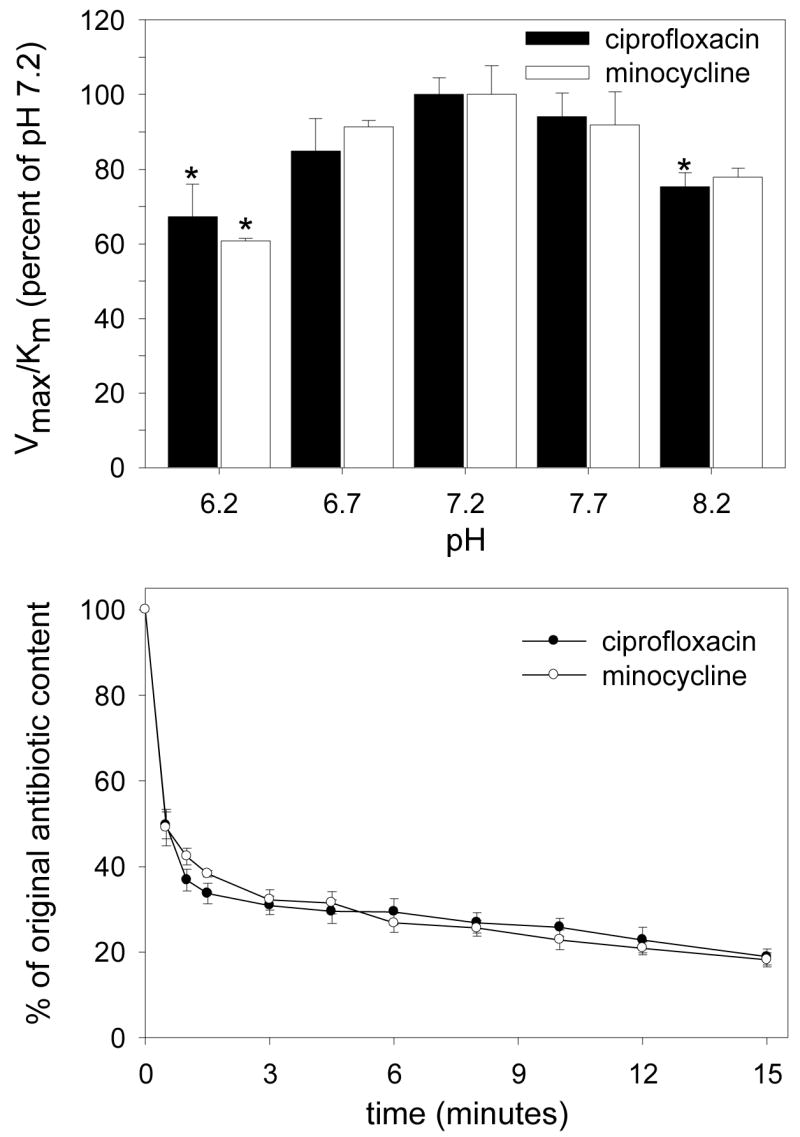
The pH-dependence and reversibility of ciprofloxacin and minocycline transport by gingival fibroblasts. Upper panel: Confluent cell monolayers were washed and overlaid with HBSS adjusted to the indicated pH. The kinetics of transport were assayed at 37° C, analyzed by the Lineweaver-Burk method and expressed as a percentage of the Vmax/Km ratio observed at pH 7.2. Data are presented as the mean ± SEM of at least 3 experiments. Changes in pH produced a significant overall effect on ciprofloxacin transport (P<0.02, ANOVA). Treatments that produced a significant difference from pH 7.2 are indicated by * (P<0.05, Dunnett’s test). Lower panel: Cell monolayers were loaded to steady-state by incubation for 20 minutes at 37° C in HBSS containing 50 μg/ml ciprofloxacin or minocycline. To trigger antibiotic efflux from the loaded cells, extracellular antimicrobial solutions were diluted 1:20 with 37° C HBSS. Efflux was monitored by the decrease in cell-associated fluorescence.
Fibroblasts loaded with ciprofloxacin or minocycline rapidly lost much of their antibiotic content when the concentration of antibiotics in the extracellular medium was abruptly diluted (Fig 2, bottom). The rate of efflux was similar for both antimicrobial agents. Approximately half the cellular antimicrobial content was lost within 45 seconds and nearly 80% was lost within 15 minutes.
Inhibition studies were performed with a variety of organic cations to determine whether the transporters of ciprofloxacin and minocycline are similar in their susceptibility to inhibition. Adenine competitively inhibited the transport of ciprofloxacin and minocycline with Ki values of 1.36 and 1.75 mm, respectively (Table 2). Diazepam failed to inhibit ciprofloxacin transport at a concentration of 2 mM, but was a potent competitive inhibitor of minocycline transport (Ki = 0.88 mM). Phenylephrine, pyrilamine, papaverine and tetracycline inhibited ciprofloxacin transport through a noncompetitive mechanism, but produced competitive inhibition of minocycline transport. Penicillin (5 mM) did not significantly inhibit the transport of ciprofloxacin or minocycline (not shown).
Table 2.
Inhibition of Ciprofloxacin and Minocycline Transport by Organic Cationsa
| Agent | Ki for ciprofloxacin transportb | Ki for minocycline transportb |
|---|---|---|
| Adenine | 1.36 ± 0.10 mM (competitive) | 1.75 ± 0.12 mM (competitive) |
| Diazepam | No inhibition at 2 mM | 0.89 ± 0.08 mM (competitive) |
| Phenylephrine | 2.66 ± 0.19 mM (noncompetitive) | 1.49 ± 0.14 mM (competitive) |
| Pyrilamine | 6.16 ± 0.45 mM (noncompetitive) | 2.76 ± 0.25 mM (competitive) |
| Papaverine | 1.74 ± 0.12 mM (noncompetitive) | 1.41 ± 0.14 mM (competitive) |
| Tetracycline | 1.76 ± 0.17 mM (noncompetitive) | 2.32 ± 0.14 mM (competitive) |
Derived from Lineweaver-Burk analysis of transport activity observed in the presence and absence of the indicated agents.
Inhibitory constants are expressed as the mean ± SEM of at least three experiments. The mechanisms of inhibition are included in parentheses.
DISCUSSION
Human gingival fibroblasts express saturable transporters that allow them to take up extracellular fluoroquinolones and tetracyclines and act as reservoirs for these agents in the gingiva. Although the affinity of forward transport is relatively low, fibroblasts accumulate remarkable amounts of minocycline and ciprofloxacin. The observed cellular/extracellular concentration (C/E) ratios were >60 for minocycline and >8 for ciprofloxacin. Consistent with these observations, the efficiency of minocycline transport (assessed by Vmax/Km) is about six times higher than for ciprofloxacin. Minocycline’s Km for transport is half that of ciprofloxacin and its transport velocity is approximately three times higher.
In addition to the differences in efficiency, transporters of ciprofloxacin and minocycline differ in their susceptibility to inhibition by other organic cations. Phenylephrine, pyrilamine, tetracycline and papaverine produced competitive inhibition of minocycline transport but acted as noncompetitive inhibitors of ciprofloxacin transport. In contrast, diazepam produced competitive inhibition of minocycline transport (Ki = 0.9 mM), but failed to inhibit ciprofloxacin transport at this concentration. Penicillin did not inhibit the transport of ciprofloxacin or minocycline. It is not preferentially concentrated in gingival fluid and does not appear to share a path of uptake with these two agents. The only agent that produced competitive inhibition of both transport processes was adenine. This suggests that transport of minocycline (which possesses primary and tertiary amines) is mediated by a system with broad substrate specificity. The organic cation transporter family may play a role, since it is widely distributed and accepts a broad range of cationic substrates (Koepsel, 1998; Dresser et al., 1999). Ciprofloxacin is a multi-ring compound that shares some structural features with purines. It could potentially interact with nucleobase or nucleoside transporters, since adenine competitively inhibits its transport. These transporters normally take up precursors for the synthesis of nucleic acids and ATP (Griffith and Jarvis, 1996).
PMNs and certain epithelial cell lines have been shown to accumulate fluoroquinolones and achieve C/E ratios similar to those observed in fibroblasts in this study (Pascual et al., 1997, 1999). Gingival fibroblasts, monocytes and resting PMNs appear to take up ciprofloxacin with similar affinity, comparable pH and sodium-dependence and identical susceptibility to competitive inhibition by adenine (Walters et al., 1999; Bounds et al., 2000). With tetracyclines, however, fibroblasts attain a C/E ratio that is at least 10-fold higher than that reported for PMNs (Gabler, 1991).
Transporters are capable of moving their substrates in the forward or reverse direction to maintain equilibrium between intracellular and extracellular concentrations. For this reason, intracellular stores of ciprofloxacin and minocycline move out of gingival fibroblasts when their concentrations decrease in the extracellular medium (Fig 2, bottom panel). During antimicrobial therapy in vivo, forward transport predominates during periods in which these agents are increasing or peaking in the blood (typically 2 to 3 hours after administration). As antibiotic levels in the blood and tissue decrease from their peak values, net transport changes to the reverse direction. Efflux from gingival fibroblasts could potentially maintain relatively high antimicrobial levels in the interstitial fluid as blood levels decrease. Due to the unique architecture of the gingiva (Schroeder and Listgarten, 1997), much of the ciprofloxacin or minocycline in interstitial fluid is eventually washed through the junctional epithelium and into the gingival crevice. The existence of a drug reservoir in the gingiva could explain why tetracycline (Gordon et al., 1981), doxycycline (Pascale et al., 1986), minocycline (Ciancio et al., 1980) and ciprofloxacin (Conway et al., 2000) appear to reach higher levels in GF than in blood serum when sampled after blood levels have receded from their peak values. It could also account for the findings of Sakellari et al. (2000), who found higher levels of tetracycline, doxycycline and minocycline in blood than in GF. Their blood and GF samples were obtained 2 hr after oral administration, which coincides with or precedes the time of peak blood levels. In summary, this study has begun to characterize transport systems that influence the effect iveness of per iodontal antimicrobial chemotherapy. The results suggest that gingival fibroblasts can function as reservoirs for fluoroquinolones and tetracyclines. Their ability to accumulate high levels of these agents may enhance their redistribution from the bloodstream to the gingiva and contribute to increased antibiotic levels in GF.
Acknowledgments
This investigation was supported by USPHS research grants DE00338 and DE12601 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
References
- Bounds SJ, Nakkula R, Walters JD. Fluoroquinolone transport by human monocytes: characterization and comparison to other cells of myeloid lineage. Antimicrob Agents Chemother. 2000;44:2609–2614. doi: 10.1128/aac.44.10.2609-2614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and convenient method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christersson LA, Albini B, Zambon JJ, Wikesjo UM, Genco RJ. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. 1. Light, immunofluorescence and culture techniques. J Periodontol. 1987;58:529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- Ciancio SG, Mather ML, McMullen JA. An evaluation of minocycline in patients with periodontal disease. J Periodontol. 1980;51:530–534. doi: 10.1902/jop.1980.51.9.530. [DOI] [PubMed] [Google Scholar]
- Conway TB, Beck FM, Walters JD. Gingival fluid ciprofloxacin levels at healthy and inflamed human periodontal sites. J Periodontol. 2000;71:1448–1452. doi: 10.1902/jop.2000.71.9.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser MJ, Zhang L, Giacomini KM. Molecular and functional characterist ics of cloned human organic cation transporters. Pharmaceutical Biotechnology. 1999;12:441–469. doi: 10.1007/0-306-46812-3_15. [DOI] [PubMed] [Google Scholar]
- Easmon CSF, Crane JP. Uptake of ciprofloxacin by human neutrophils. J Antimicrob Chemother. 1985;16:67–73. doi: 10.1093/jac/16.1.67. [DOI] [PubMed] [Google Scholar]
- Fives-Taylor P, Meyer D, Mintz K. Virulence factors of the periodontopathogen Actinobacillus actinomycetemcomitans. J Periodontol. 1996;67:291–297. doi: 10.1902/jop.1996.67.3s.291. [DOI] [PubMed] [Google Scholar]
- Gabler WL. Fluxes and accumulation of tetracyclines by human blood cells. Res Commun Chemical Pathol Pharmacol. 1991;72:39–51. [PubMed] [Google Scholar]
- Garraffo R, Jambou D, Chichmanian RM, Ravoire S, Lapalus P. In vitro and in vivo ciprofloxacin pharmacokinetics in human neutrophils. Antimicrob Agents Chemother. 1991;35:2215–2218. doi: 10.1128/aac.35.11.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola GC, Dall’Asta V, Franchi-Gazzola R, White MF. The cluster-tray method for rapid measurement of solute fluxes in adherent cultured cells. Anal Biochem. 1981;115:368–374. doi: 10.1016/0003-2697(81)90019-1. [DOI] [PubMed] [Google Scholar]
- Gordon JM, Walker CB, Murphy JC, Goodson JM, Socransky SS. Concentration of tetracycline in human gingival fluid after single doses. J Clin Periodontol. 1981;8:117–121. doi: 10.1111/j.1600-051x.1981.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Griffith DA, Jarvis SM. Nucleoside and nucleobase transport systems o f mammalian cells. Biochem Biophys Acta. 1996;1286:153–181. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Koepsel H. Organic cation transporters in intestine, kidney, liver and brain. Annu Rev Physiol. 1998;60:243–266. doi: 10.1146/annurev.physiol.60.1.243. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Chan A, Belton CM, Izutsu K, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epit helial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. Improved fluorometric determination of tetracyclines. Biochem Med. 1972;6:216–222. doi: 10.1016/0006-2944(72)90041-5. [DOI] [PubMed] [Google Scholar]
- Mariotti A, Cochran DL. Characterization of fibroblasts derived from human periodontal ligament and gingiva. J Periodontol. 1990;61:103–111. doi: 10.1902/jop.1990.61.2.103. [DOI] [PubMed] [Google Scholar]
- Pascale D, Gordon J, Lamster I, Mann P, Seiger M, Arndt W. Concentration of doxycycline in human gingival fluid. J Clin Periodontol. 1986;13:841–844. doi: 10.1111/j.1600-051x.1986.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Pascual A, Garcia I, Ballesta S, Perea EJ. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1997;41:274–277. doi: 10.1128/aac.41.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Garcia I, Ballesta S, Perea EJ. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1999;43:12–15. doi: 10.1128/aac.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavicic MJ, van Winkelhoff AJ, deGraaff J. In vitro susceptibilities of Actinobacillus actinomycetemcomitans to a number of antimicrobial combinations. Antimicrob Agents Chemother. 1992;36:2634–2638. doi: 10.1128/aac.36.12.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea EJ, Garcia I, Pascual A. Comparative penetration of lomefloxacin and other quinolones into human phagocytes. Am J Med. 1992;92(suppl 4A):48–51. doi: 10.1016/0002-9343(92)90309-y. [DOI] [PubMed] [Google Scholar]
- Sakellari D, Goodson JM, Socransky SS, Kolokotronis A, Konstantinidis A. Concentration of 3 tetracyclines in plasma, gingival crevice fluid and saliva. J Clin Periodontol. 2000;27:53–60. doi: 10.1034/j.1600-051x.2000.027001053.x. [DOI] [PubMed] [Google Scholar]
- Schroeder HE, Listgarten MA. The gingival tissues: the archit ecture o f periodontal protection. Periodontol 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontology 2000. 1996;10:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Walters JD, Zhang F, Nakkula RJ. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob Agents Chemother. 1999;43:2710–2715. doi: 10.1128/aac.43.11.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]


