Abstract
White blood cells (WBCs) were counted in 4697 individuals who presented to outpatient malaria clinics in Maesod, Tak Province, Thailand, and Iquitos, Peru, between 28 May and 28 August 1998 and between 17 May and 9 July 1999. At each site and in each year, WBC counts in the Plasmodium falciparum–infected patients were lower than those in the Plasmodium vivax–infected patients, which, in turn, were lower than those in the uninfected patients. In Thailand, one-sixth of the P. falciparum–infected patients had WBC counts of <4000 cells/μL. Leukopenia may confound population studies that estimate parasite densities on the basis of an assumed WBC count of 8000 cells/μL. For instance, in the present study, use of this conventional approach would have overestimated average asexual parasite densities in the P. falciparum–infected patients in Thailand by nearly one-third.
White blood cell (WBC) counts during malaria are generally characterized as being low to normal, a phenomenon that is widely thought to reflect localization of leukocytes away from the peripheral circulation and to the spleen and other marginal pools, rather than actual depletion or stasis. Leukocytosis is typically reported in a fraction of cases and may be associated with concurrent infections and/or poor prognosis. Remarkably few published studies have compared WBC counts in malarial parasite–infected and –uninfected residents of regions in which malaria is endemic, however.
Human malaria can be caused by any of several species of Plasmodium parasites that occur together in various combinations in regions of endemicity. P. falciparum is responsible for almost all mortality attributed directly to malaria and is the focus of almost all research and intervention efforts. Compared with P. falciparum, however, P. vivax is the source of as much or more morbidity worldwide, despite its extremely low prevalence in sub-Saharan Africa. The tacit assumption that WBC counts are identical during infections with different Plasmodium species has been examined only minimally and tangentially.
Although several methods for estimation of densities of blood-stage parasites by microscopy are in use, the most common is to count the number of asexual parasites seen relative to a given count of WBCs (usually 200 or 500 cells) and then to multiply the parasite: WBC ratio by 8000, the assumed number of WBCs per microliter of blood. These estimates are used in clinical and epidemiological studies and in evaluation of the effects of interventions on individuals and communities. The consequences of errors are strongly dependent on context but could be profound, as would be the case in studies that relate malarial symptoms or transmission to parasite densities.
PATIENTS, MATERIALS, AND METHODS
Microscopy data were collected during a study of rapid diagnostic devices for malaria, for which the methods are described in detail elsewhere [1–3]. Study participants were symptomatic patients who presented of their own initiative to existing outpatient malaria clinics operated by local public health authorities. During the study, the staff of these clinics retained full responsibility for patient care; all diagnostic and treatment decisions were made independent of the study protocol. The study protocol was reviewed by the Institutional Review Board of the Walter Reed Army Institute of Research and the Human Subjects Safety Review Board of the US Army Medical Research and Materiel Command and was approved as Walter Reed Army Institute of Research Protocol 687 (version 2.21, 1998; version 2.3, 1999). The protocol was approved annually by the Ministry of Health in Iquitos, Peru, and was performed under the direction of the Dirección de Salud de Loreto. Similarly, it was approved by the Thai Ministry of Public Health for each year of implementation and was operated with oversight from Vector-Borne Disease Control Office No. 1, Phrabuddhabat, Saraburi Province, Thailand.
Briefly, the participants were those who presented to local malaria clinics in Maesod, Tak Province, Thailand, and in Iquitos, Peru, between 28 May and 28 August 1998 or between 17 May and 9 July 1999; were ≥15 years old (Thailand) or ≥1 year old (Peru); and had fever (oral temperature of ≥38°C), headache, or a self-reported history of fever within the previous 72 h. The oral temperature of each patient was taken immediately before blood was obtained. In Thailand, 66% of patients in 1998 and 70% of patients in 1999 were male; in Peru, 56% of patients were male in each year. The median self-reported age of patients in Thailand was 24 years in 1998 and 25 years in 1999; the corresponding figures in Peru were 26 and 31 years. Differences in age distribution between 1998 and 1999 were of marginal significance (for Thailand, P = .035; for Peru, P = .055; Mann-Whitney U test), although no patients <18 years old enrolled at either site in 1999 (vs. 8% in Thailand and 27% in Peru in 1998). If patients <18 years old are removed from the 1998 data before the 2 years are compared, the age distributions of the populations are statistically indistinguishable (for Thailand, P = .07; for Peru, P = .10; Mann-Whitney U test). At presentation, patients were asked the number of days they had felt ill; responses were recorded in days from 1 to 8, with 8 representing >7 days. Patients were also asked whether they had taken antimalarial drugs within the previous 2 weeks; those who gave positive responses were excluded from the present analysis.
Approximately 2 mL of venous blood was drawn from each patient into an EDTA-filled tube, to be used for blood films and an automated WBC count. Precise volumes of well-mixed whole blood were micropipetted and prepared as thick and thin smears on each of 3 precleaned slides by a team of well-trained technicians following a standardized procedure. One slide was used promptly by the local clinic staff for diagnosis and medical intervention, if indicated. The 2 remaining slides were held overnight and then stained with 3% Giemsa. One of these slides was read by each of 2 expert microscopists, who were blinded to the other’s interpretation. Two hundred oil-immersion high-power fields were read on the thick smear before any slide was interpreted as being negative; the thin smear was used only for determination of species. Identification of species and estimates of parasite density were based solely on parasite asexual blood forms. If, after 200 WBCs were counted, ≥10 asexual parasite stages had been counted, the total number of asexual parasites was recorded; if, after 200 WBCs were counted, parasites were present but numbered <10 asexual parasite stages, then the microscopists continued to examine the smear, counting asexual parasites and WBCs until 500 WBCs had been counted. If the reports of the microscopists disagreed on the presence or species of parasites or on parasite density by a factor of ≥2, a senior microscopist examined both slides. (As a quality-control measure, a senior microscopist also reviewed both slides for 5% of the cases on which the 2 initial microscopists agreed.) The 4697 cases considered here exclude the 94 cases on which the 2 initial microscopists disagreed with respect to the presence or species of parasites and the 24 cases on which they agreed with respect to the infections being of mixed species. The parasite densities used here are the average of the estimates from the 2 initial microscopists.
Blood tubes were transported on ice within 2 h to a field laboratory, and, within 6 h of the initial sample collection, WBCs were counted by use of a Coulter automated cell counter (Beckman-Coulter) in Thailand and a QBC centrifugal hematology system (Becton-Dickinson Diagnostic Systems) in Peru. To validate WBC counts, daily quality-assurance checks and additional replicate testing were performed; calibrated commercial standards were used in accordance with the manufacturers’ recommendations. To provide an external monitor of instrument function, WBCs were counted manually on a weekly basis throughout the study, using prepared dilutions. The QBC system used in Peru is not as accurate as the Coulter system used in Thailand, however; in particular, in the QBC system, infected red blood cells (RBCs) may float into the WBC layer and be misidentified as WBCs. This is especially likely with P. vivax–infected RBCs, because their trophozoites are often more buoyant than P. falciparum ring forms (the only P. falciparum asexual forms typically seen in the peripheral blood); as is noted below, this can lead to artificially increased WBC counts in individuals with higher parasite densities.
As was expected, the data on WBC counts had a skewed distribution, with a tail of higher values [4]. We compared distributions by the Mann-Whitney U and Kolmogorov-Smirnov tests, and we related WBC counts to parasite densities by Spearman’s rank correlation coefficient and the slope of the least-squares best fit linear regression line [5]. The Kolmogorov-Smirnov test measures differences in the shape (dispersion and skewness) as well as the location of the entire distribution and so is less sensitive to differences in location than the Mann-Whitney U test. All P values given are for 2-tailed tests. To investigate trends in the data on the number of days ill, we performed a runs test on the sign of the difference between consecutive values [5].
RESULTS
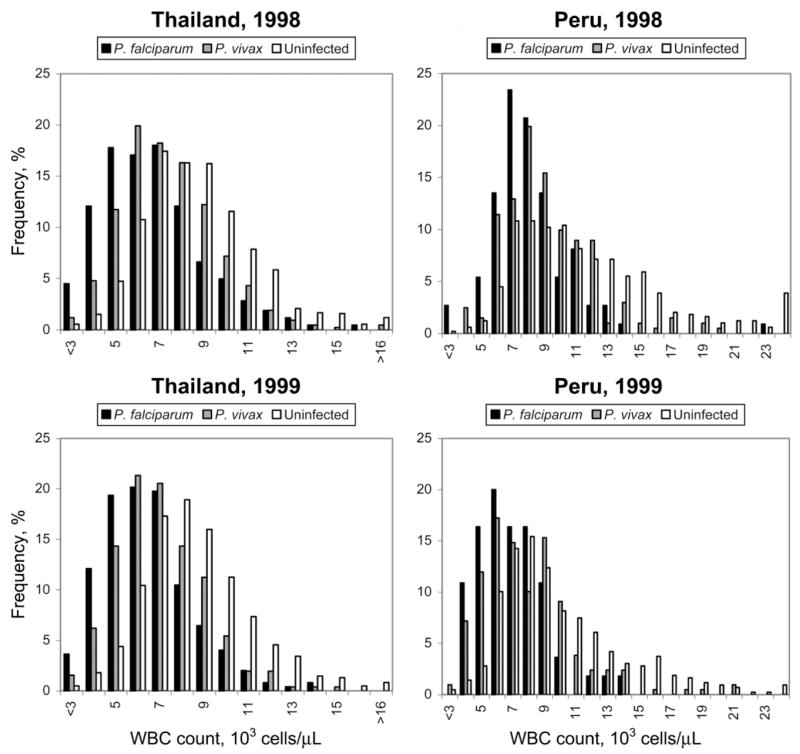
Figure 1 shows histograms of the WBC counts for the uninfected, the P. vivax–infected, and the P. falciparum–infected patients at the clinics in Thailand and Peru in 1998 and 1999. The WBC counts in the P. falciparum–infected patients were lower than those in the P. vivax–infected patients, and the WBC counts in the P. vivax–infected patients were lower than those in the uninfected patients. These differences were apparent at each site and in each year and were confirmed by statistical tests. We compared the distributions of the WBC counts within each site and each year for the P. falciparum–infected, P. vivax–infected, and uninfected patients. In 11 of the 12 Mann-Whitney U tests and in 9 of the 12 Kolmogorov-Smirnov tests, including all of the tests for comparisons between the P. falciparum–infected patients and the uninfected patients and between the P. vivax–infected patients and the uninfected patients, the P values were <.000001. The largest P value from the Mann-Whitney U tests was .0003, for the comparison between the P. falciparum–infected and the P. vivax–infected patients in Thailand in 1999. The largest P values from the Kolmogorov-Smirnov tests were also for comparisons between the P. falciparum–infected and the P. vivax–infected patients: for the data from Peru in 1999, P = .03; for the data from Thailand in 1999, P = .008; and for the data from Peru in 1998, P = .0003.
Figure 1.
Frequency of white blood cell (WBC) counts in uninfected, Plasmodium vivax–infected, and Plasmodium falciparum–infected patients at clinics in Thailand and Peru in 1998 and 1999.
The differences in WBC count were not simply a function of asexual parasite density. Table 1 gives median values and 95% confidence limits for WBC counts, parasite densities, and temperatures. In Thailand in both 1998 and 1999, the parasite densities during P. falciparum infections were higher than those during P. vivax infections—the P values from the Mann-Whitney U tests comparing P. falciparum and P. vivax densities in Thailand were<.00001—but this was not the case in Peru: the P values were .05 for 1998 and>.5 for 1999. Furthermore, the relationship between WBC count and parasite density showed no consistent pattern when considered across sites, years, and infecting species. Table 2 gives the Spearman’s rank correlation coefficients and the slopes of the least-squares best fit linear regression lines for the relationships between log WBC counts and log parasite densities. In 7 of the 8 site/year/infectingspecies combinations, the slope was positive, and, in 5 of the 8 combinations, the correlation was significant at the P < .01 level: 2 in Thailand and 3 in Peru; 2 in 1998 and 3 in 1999; and 2 for P. falciparum infection and 3 for P. vivax infection. (As is noted above, the strength of the relationship between WBC count and P. vivax density in Peru must be interpreted with caution.) When the infecting species were not considered separately, the slope was always positive, and the correlation was significant at the P < .01 level in Peru only (as would be expected, given the similarity in parasite density between the infecting species). Thus, WBC counts were higher, and parasite densities were lower (nominally zero), in the uninfected patients, compared with those in the infected patients, and WBC counts were higher in the P. vivax–infected patients, compared with those in the P. falciparum–infected patients, independent of parasite density. Correlations between WBC count and parasite density were generally positive and, unlike the differences in WBC count, were irregularly distributed across sites, years, and infecting species.
Table 1.
Median values for white blood cell (WBC) counts, parasite densities, and temperature in uninfected, Plasmodium vivax–infected, and Plasmodium falciparum–infected patients.
| Location, year | WBC count, cells/μL | Parasite density, log10 parasites/μL | Temperature, °C |
|---|---|---|---|
| Thailand | |||
| 1998 | |||
| Uninfected | 7900 (7600–8100) | … | 37.5 (37.5–37.6) |
| P. vivax | 6700 (6300–7100) | 3.43 (3.30–3.56) | 37.9 (37.8–38.1) |
| P. falciparum | 5850 (5400–6200) | 3.98 (3.83–4.09) | 38.3 (38.0–38.6) |
| 1999 | |||
| Uninfected | 7700 (7400–8000) | … | 37.3 (37.3–37.4) |
| P. vivax | 6250 (5900–6700) | 3.53 (3.23–3.68) | 37.8 (37.5–38.2) |
| P. falciparum | 5600 (5200–6000) | 4.01 (3.77–4.20) | 38.1 (37.7–38.5) |
| Peru | |||
| 1998 | |||
| Uninfected | 10,100 (9500–10,800) | … | 37.4 (37.2–37.5) |
| P. vivax | 8000 (7600–8600) | 3.74 (3.63–3.97) | 37.7 (37.4–38.1) |
| P. falciparum | 7100 (6600–7600) | 3.94 (3.69–4.20) | 37.7 (37.5–38.1) |
| 1999 | |||
| Uninfected | 8300 (7800–9000) | … | 37.1 (37.0–37.2) |
| P. vivax | 6900 (6100–7600) | 3.64 (3.40–3.79) | 37.4 (37.1–37.9) |
| P. falciparum | 6000 (5000–7100) | 3.72 (3.26–4.09) | 37.7 (37.1–38.4) |
NOTE. Data are median values (95% confidence limits).
Table 2.
Relationships between white blood cell (WBC) counts and parasite densities.
| Thailand
|
Peru
|
|||
|---|---|---|---|---|
| Pathogen | 1998 | 1999 | 1998 | 1999 |
| Plasmodium falciparum | 0.009; 0.104 | 0.018; 0.170a | −0.006; 0.051 | 0.056; 0.357a |
| Plasmodium vivax | 0.010; 0.139a | 0.004; 0.113 | 0.067; 0.343b | 0.089; 0.462b |
| Combined | 0.0004; 0.039 | 0.0056; 0.071 | 0.0330; 0.201c | 0.0787; 0.415b |
NOTE. Data are (at left) the slopes of the least-squares best fit linear regression lines for the relationships between log WBC counts and log parasite densities and (at right) Spearman’s rank correlation coefficients.
P < .01.
P < .00001.
P < .001.
The differences in WBC count were not consistent with the pattern of temperatures, either (table 1). The temperature distributions differed dramatically between the uninfected patients and the infected patients at each site and in each year, whether the infecting species were considered separately or together: all P values from the Mann-Whitney U tests and the Kolmogorov-Smirnov tests were <.0001, except for those for Peru in 1998, for which the P values were <.001. The temperature distributions generally did not differ with the infecting species, however: all P values were >.5, except for that for Thailand in 1998, for which P = .003. For P. falciparum infections, the slope of the relationship between WBC count and temperature was always positive, and the P values from the Spearman’s rank correlations ranged from 0.03 to 0.20; the slope was always negative for P. vivax infections and when the infecting species were not considered separately, and all P values were >.4. The slope for the relationship between WBC count and temperature was strongly positive for uninfected patients, with P = .03 for Thailand in 1998, P = .0004 for Thailand in 1999, and P < .000001 for Peru in both 1998 and 1999; this pattern may reflect bacterial infections of differing severity. There was a strong positive relationship between temperature and parasite density: all P values from the Spearman’s rank correlations were <.0001, except for that for P. falciparum infection in Peru in 1999, for which P = .01. The lack of serial blood collection and temperature measurements for each patient limited our capacity to study temperature relationships, however, as did the possibility that patients self-medicated with antipyretics.
There was no trend in the median WBC counts with respect to the reported number of days ill: only 1 of the 12 runs tests (for P. vivax infection in Thailand in 1998) was significant at the P = .05 level. Similarly, for median parasite density and temperature (considered both independently and matched to the median WBC count and/or parasite density), only 4 of the 48 runs tests were significant at the P = .05 level. The distribution of the number of patients over the reported number of days ill was highly heterogeneous: overall, 80% of the patients were ill for 1–4 days, but the percentage who were ill for 2–3 days ranged from 38% (in Peru in 1999) to 68% (in Thailand in 1999). These patterns may reflect differences in access to health-care facilities at the sites in each year. Nonetheless, in 29 of the 32 site/year/days ill combinations, the median WBC counts showed the same pattern as in the aggregate data: higher in the uninfected patients than in the P. vivax–infected patients and higher in the P. vivax–patients than in the P. falciparum–infected patients. In the other 3 combinations, the median WBC counts for P. falciparum–infected patients were higher than those for P. vivax–infected patients, but there were ≤6 patients in 1 or both categories.
Table 1 shows that median WBC counts in Peru were greater than those in Thailand, in both 1998 and 1999 and in all 3 categories of patients (uninfected, infected with P. falciparum, and infected with P. vivax). In general, this pattern held when we compared only male patients to male patients and only female patients to female patients, but it was reversed in female patients infected with either P. falciparum or P. vivax in 1999. Because differences in equipment between the sites may have confounded these differences (see Patients, Materials, and Methods), we did not pursue this analysis.
Table 1 also shows that the median WBC counts in 1999 were lower than those in 1998 at both sites and for all 3 categories of patients. The difference was clearly significant for all 3 categories of patients in Peru (P < .0006, Mann-Whitney U test) and was marginally significant in the P. vivax–infected patients in Thailand (P = .025, Mann-Whitney U test). In white populations, WBC counts typically decrease with age and are lower in male adults than in female adults [4, 6]. In our data, among the uninfected patients in Peru and the P. vivax–infected patients in Thailand, the differences in median WBC counts between 1998 and 1999 were associated with differences in sex ratios and/or age distribution. This did not apply to the P. falciparum– and P. vivax–infected patients in Peru, however; these differences were associated with steep decreases in WBC counts in female patients, a finding that we cannot explain (see the Appendix).
DISCUSSION
Our analysis of WBC counts in individuals presenting at Thai and Peruvian outpatient malaria clinics in 1998 and 1999 showed that the P. falciparum–infected patients had lower WBC counts than the P. vivax–infected patients and that both of these groups of patients had lower WBC counts than the uninfected patients. Two previous studies, both in Asia [7, 8], have reported WBC counts during P. falciparum and P. vivax infections in residents of regions in which Plasmodium parasites are endemic; to the best of our knowledge, the present study is the first such report from a South American site.
One of the 2 previous studies comparing WBC counts in P. falciparum– and P. vivax–infected residents of a region of endemicity—at a hospital outpatient clinic in India—found no difference between mean WBC counts [7]. We cannot explain the discrepancy. This study reported WBC counts of <4000 cells/mm3 (its definition of leukopenia) in 10.7% of 112 P. falciparum–infected patients and in 15.2% of 118 P. vivax–infected patients. In our data, WBC counts of <4000 cells/μL (or of less than any cutoff value up to 10,000 cells/μL) were more frequent in P. falciparum–infected patients than in P. vivax–infected patients (table 3). The authors of the India study did not describe the methods they used for determination of WBC counts, and they did use statistical tests that assume Gaussian distributions of data; many other factors may be involved as well. Another study [9] reported that leukopenia (defined as WBC counts of <5000 cells/mm3) was more common during P. falciparum infection than during P. vivax infection among 81 US servicemen in Vietnam. Two case series from industrialized countries that included comparative information reported that WBC counts during P. falciparum and P. vivax infections [10] and during “non-falciparum” infections [11] did not appreciably differ.
Table 3.
Frequency of white blood cell counts of <4000 cells/μL and of >16,000 cells/μL in uninfected, Plasmodium vivax–infected, and Plasmodium falciparum–infected patients.
| Location | <4000 cells/μL | >16,000 cells/μL |
|---|---|---|
| Thailand, 1998–1999 | ||
| Uninfected | 40 (2.1) | 20 (1.1) |
| P. vivax | 45 (6.7) | 2 (0.3) |
| P. falciparum | 109 (16.3) | 0 (0.0) |
| Peru, 1998–1999 | ||
| Uninfected | 12 (1.3) | 99 (10.8) |
| P. vivax | 22 (5.4) | 10 (2.4) |
| P. falciparum | 9 (5.4) | 1 (0.6) |
NOTE. Data are no. (%) of patients.
For each infecting species, at each site, and in each year, we found a consistent positive relationship between WBC count and parasite density in the Plasmodium-infected patients in the present study, although the strength and putative statistical significance of the relationship varied. As the data in table 2 suggest, the relationship was pronounced only in those instances in which it was most likely to have been exaggerated by a technical artifact. The one previous study that examined WBC count in relation to parasite density during P. falciparum and P. vivax infections in a region in which Plasmodium parasites were endemic was also conducted in western Thailand, and it reported that there was no trend across quartiles of parasite density [8]. These results do not conflict: our data from Thailand show only 1 strong WBC count relationship for each year, with a different species in each year. Furthermore, due to reenforcement of Thai immigration law, the 2001 study population used in this previous study may have been different from our 1998 and 1999 study populations in terms of baseline health and nutritional status as well as treatment-seeking behavior.
The lower median WBC counts in 1999 in the P. vivax–infected patients in Thailand and the uninfected patients in Peru may be attributable to differences in sex ratio and/or age distribution between the 1998 and 1999 study populations. These associations did not hold for the lower median WBC counts in the P. falciparum–infected patients and the P. vivax–infected patients in Peru, however, although the differences arose from the female patients; we cannot explain why their WBC counts decreased in 1999, to far below the WBC counts in male patients. We hope that future studies will provide more insight into sources of WBC count variation, including information on the dynamics of specific WBC types in the peripheral circulation and underlying pathophysiological mechanisms.
Wide variation is to be expected in any such observations, given the enormous number of factors involved. For instance, even within the rather restricted sample considered here, variation might have reflected the timing of observations and the clinical status of infections; the statics seen in a cross-sectional survey may not fully reflect underlying dynamics [12]. Early researchers established the association between peripheral leukopenia and malaria [13] and between peripheral leukocytosis and severe cases [14]. They observed that, during initial infection, the WBC count decreases to its minimum at roughly the same time that fever begins and infection becomes detectable by microscopy; this seems to be in accordance with the findings of the 2 more-recent reports [15, 16], although neither of these involved naturally acquired infections. Many early researchers also held that leukopenia, even in mild cases, is punctuated by a transient leukocytosis, either during [17] or between [18] febrile paroxysms; this view was debated for decades [19] and is echoed in some modern texts [20]. Our results here argue against such connections, but we look forward to the results of future studies that have greater temporal resolution and that are conducted in different populations. There has been some revival of interest in the prognostic value of leukocytosis (studies have been conducted in P. falciparum–infected African children [21, 22]), but the scarcity of recent studies of WBC dynamics and their clinical correlates is remarkable.
Still more remarkable is the degree to which the estimation of blood-stage parasite densities by microscopy has become dominated by the convenient but inaccurate assumption of a constant WBC count of 8000 cells/μL of peripheral blood [23, 24]. A simple, reliable, well-known alternative does not depend on WBC counts at all but only on keeping the volume of blood on thick films fairly consistent [25]. Defending the more common method, Payne wrote that variation in estimated parasite density by “a factor of 1 either way” (i.e., 50%–200% of the true density), on the basis of similar variation in WBC count, “is not of significance to the physician or the epidemiologist” ([26], p. 623). Warhurst and Williams [27] have suggested that an overestimate of WBC density might balance the loss of parasites that can occur during the preparation of a thick film [28]. Indeed, the common method works well in many—and perhaps most—circumstances, but its use must be questioned in situations in which parasite density is the basis of a research hypothesis or an end-point evaluation in an intervention trial [29]. Leukopenia may confound such studies. For instance, in our data sets, one-sixth of the P. falciparum–infected patients in Thailand had WBC counts of <4000 cells/μL; had we used the conventional assumption of 8000 WBCs/μL rather than actually counting WBCs, we would have overestimated average parasite density in these patients by nearly one-third.
In any scientific study, failure to recognize the limits of accuracy of the observations renders interpretation problematic. Microscopy pervades studies of malaria and is used as the reference for evaluation of new diagnostic tests, such as antigen detection and semiquantitative polymerase chain reaction techniques. However, valid comparisons require accurate parasite counts. It is important to determine threshold levels of parasite densities at which the accuracy of new diagnostic devices diminishes; for instance, ascertainment of such thresholds is critical to assessment of the safety of their use. Accurate parasite-clearance profiles are useful for therapeutic monitoring, particularly in situations of widespread drug resistance. Many epidemiological studies rely on microscopy-based estimates of parasite density, stratified by age or other factors. Our present results add to the strong but neglected case for improving the standard methods of malaria microscopy.
Acknowledgments
We thank the Armed Forces Research Institute of Medical Sciences and US Naval Medical Research Center Detachment field study teams, for their dedication and technical assistance, and gratefully acknowledge the contributions of M. Fukuda, R. Luther, D. Mason, J. Muench, M. Webb, and 2 anonymous reviewers.
Financial support: US Army Medical Research and Materiel Command.
APPENDIX
In each category of patient at each site, male participation differed between 1998 and 1999 by only 1%–3%, with the exception of P. vivax–infected patients in Thailand (in 1998, 69% were male; in 1999, 76% were male). There were notable differences in WBC count between male and female patients only in those infected with P. falciparum (6550 vs. 4800 cells/μL; P = .003, Mann-Whitney U test) or with P. vivax (8000 vs. 6000 cells/μL; P = .0001, Mann-Whitney U test) in Peru in 1999. In Peru, same-sex comparisons showed strong differences in WBC count between 1998 and 1999 (P < .0002, Mann-Whitney U test) in uninfected patients of both sexes and in P. falciparum– and P. vivax–infected female patients. In Thailand, the only notable difference between 1998 and 1999 was in P. vivax–infected male patients (P = .01, Mann-Whitney U test).
In each category, at each site, and in each year, WBC counts decreased with age (the slope of the least-squares best fit linear regression line was negative). The Spearman’s rank correlation was significant at the P < .01 level in uninfected patients at both sites in both 1998 and 1999, in P. falciparum–infected patients at both sites in 1998, and in P. vivax–infected patients in Thailand in 1998. If participants <18 years old are removed from the 1998 data before the 2 years are compared, the Mann-Whitney U test P value for the P. vivax–infected patients in Thailand increases to .09, but the P values remain <.002 for all 3 categories of patients in Peru. Following through with same-sex comparisons between 1998 and 1999, the P values for the P. vivax–infected male and female patients in Thailand were .02 and .64, respectively. In Peru, the P values increased to >.05 for the uninfected patients of both sexes and for the P. falciparum– and the P. vivax–infected male patients but remained <.0005 for the P. falciparum– and the P. vivax–infected female patients. The age distributions of the P. falciparum– and the P. vivax–infected female patients in Peru did not differ between 1998 and 1999 (P > .46, Mann-Whitney U test). Thus, the difference in WBC count between 1998 and 1999 in the P. vivax–infected patients in Thailand appears to be associated primarily with a difference in age distribution (influenced by sex ratio), and the difference in WBC count between 1998 and 1999 in the uninfected patients in Peru appears to be associated with a combined difference in sex ratio and age distribution.
There were no notable differences in P. falciparum or P. vivax density between 1998 and 1999 at either site (P > .08, Mann-Whitney U test) or between parasite density in the P. falciparum– or the P. vivax–infected male and female patients at either site in either year (P > .06, Mann-Whitney U test). In same-sex comparisons of parasite density between 1998 and 1999, all P values were >.07, except for the P. vivax–infected female patients in Thailand (P = .04). The different intervals of enrollment and clustering of patient enrollment dates may have had an effect (the median calendar dates of enrollment were 14 July in Thailand and 12 July in Peru in 1998; the corresponding dates in 1999 were 22 June and 3 June), but we lack data on corresponding differences in local biological or epidemiological variables.
Footnotes
The views presented here are those of the authors and are not to be taken to represent those of our respective institutions.
This article is in the public domain, and no copyright is claimed.
References
- 1.Forney JR, Magill AJ, Wongsrichanalai C, et al. Malaria rapid diagnostic devices: performance characteristics of the ParaSight F device determined in a multisite field study. J Clin Microbiol. 2001;39:2884–90. doi: 10.1128/JCM.39.8.2884-2890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forney JR, Wongsrichanalai C, Magill AJ, et al. Devices for rapid diagnosis of malaria: evaluation of prototype assays that detect Plasmodium falciparum histidine-rich protein 2 and a Plasmodium vivax-specific antigen. J Clin Microbiol. 2003;41:2358–66. doi: 10.1128/JCM.41.6.2358-2366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenzie FE, Sirichaisinthop J, Miller RS, Gasser RA, Jr, Wongsrichanalai C. Dependence of malaria detection and species diagnosis by microscopy on parasite density. Am J Trop Med Hyg. 2003;69:372–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Bain BJ. Blood cells. Oxford: Blackwell Science; 2002. [Google Scholar]
- 5.Sokal RR, Rohlf FJ. Biometry. New York: WH Freeman; 1981. [Google Scholar]
- 6.Lentner C, editor. Geigy scientific tables. Vol. 3. Basel: Ciba-Geigy; 1984. [Google Scholar]
- 7.Jadhav UM, Singhvi R, Shah R. Prognostic implications of white cell differential count and white cell morphology in malaria. J Postgrad Med. 2003;49:218–21. [PubMed] [Google Scholar]
- 8.Erhart LM, Yingyuen K, Chuanak N, et al. Hematologic and clinical indices of malaria in a semi-immune population of western Thailand. Am J Trop Med Hyg. 2004;70:8–14. [PubMed] [Google Scholar]
- 9.Goldstein E. A clinical study of falciparum and vivax malaria in Vietnam servicemen. Mil Med. 1968;133:991–6. [PubMed] [Google Scholar]
- 10.Reiley CG, Barrett O., Jr Leucocyte response in acute malaria. Am J Med Sci. 1971;262:153–8. doi: 10.1097/00000441-197109000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson B, Hellgren U, Rombo L. Changes in erythrocyte sedimentation rate, C-reactive protein and hematological parameters in patients with acute malaria. Scand J Infect Dis. 1989;21:434–41. [PubMed] [Google Scholar]
- 12.Becker NG. Analysis of infectious disease data. London: Chapman and Hall; 1989. [Google Scholar]
- 13.Marchiafava E, Bignami A. On summer-autumn malarial fevers. London: The New Syndenham Society; 1894. [translated from the Italian first ed. by J Harry Thompson] [Google Scholar]
- 14.Deaderick WH. A practical study of malaria. Philadelphia: WB Saunders; 1909. [Google Scholar]
- 15.Rzepczyk CM, Stamatiou S, Anderson K, et al. Experimental human Plasmodium falciparum infections: longitudinal analysis of lymphocyte responses with particular reference to gamma delta T cells. Scand J Immunol. 1996;43:219–27. doi: 10.1046/j.1365-3083.1996.d01-24.x. [DOI] [PubMed] [Google Scholar]
- 16.Church LW, Le TP, Bryan JP, et al. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis. 1997;175:915–20. doi: 10.1086/513990. [DOI] [PubMed] [Google Scholar]
- 17.Stephens JWW, Christophers SR. The practical study of malaria and other blood parasites. 3. London: University Press of Liverpool; 1908. [Google Scholar]
- 18.Ross R, Thomson D. Some enumerative studies on malarial fever. Ann Trop Med Parasitol. 1910;4:267–81. [Google Scholar]
- 19.Taliaferro WH. Immunity to the malaria infections. In: Boyd MF, editor. Malariology. Philadelphia: WB Saunders; 1949. pp. 935–65. [Google Scholar]
- 20.Markell EK, John DT, Krotoski WA. Medical parasitology. Philadelphia: WB Saunders; 1999. [Google Scholar]
- 21.Modiano D, Sirima BS, Konate A, Sanou I, Sawadogo A. Leucocytosis in severe malaria. Trans R Soc Trop Med Hyg. 2001;95:175–6. doi: 10.1016/s0035-9203(01)90152-x. [DOI] [PubMed] [Google Scholar]
- 22.Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119:839–47. doi: 10.1046/j.1365-2141.2002.03904.x. [DOI] [PubMed] [Google Scholar]
- 23.Swan JM. A contribution to the question of the leucocyte formula in malaria. Am J Trop Med Hyg. 1922;2:283–8. [Google Scholar]
- 24.Greenwood BM, Armstrong JRM. Comparison of two simple methods for determining malaria parasite densities. Trans Roy Soc Trop Med Hyg. 1991;85:186–8. doi: 10.1016/0035-9203(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 25.Earle WC, Perez M. Enumeration of parasites in the blood of malarial patients. J Lab Clin Med. 1932;17:1124–30. [Google Scholar]
- 26.Payne D. Use and limitations of light microscopy for diagnosing malaria at the primary health level. Bull World Health Organ. 1988;66:621–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Warhurst DC, Williams JE. Laboratory diagnosis of malaria. J Clin Pathol. 1996;49:533–8. doi: 10.1136/jcp.49.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowling MAC, Shute GT. A comparative study of thick and thin films in the diagnosis of scanty malaria parasitemia. Bull World Health Organ. 1966;34:249–67. [PMC free article] [PubMed] [Google Scholar]
- 29.Ohrt C, Purnomo, Sutamihardja MA, Tang D, Kain KC. Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J Infect Dis. 2002;186:540–6. doi: 10.1086/341938. [DOI] [PubMed] [Google Scholar]