Abstract
Background
Clinical symptoms of mixed-species malaria infections have been variously reported as both less severe and more severe than those of single-species infections.
Methods
Oral temperatures were taken from and blood slides were prepared for 2308 adults who presented at outpatient malaria clinics in Tak Province (Thailand) during May–August 1998, May–July 1999, and May–June 2001 with malaria infections diagnosed by 2 expert research microscopists, each of whom was blinded to the other’s reports.
Results
In each year, temperatures of patients with mixed Plasmodium vivax–Plasmodium falciparum infections were higher than temperatures of patients with P. vivax or P. falciparum infections. In every mixed-species case, P. falciparum parasitemia was higher than P. vivax parasitemia, but patient temperature was not correlated with the parasitemia of either species or with the total parasitemia.
Conclusions
Among adults who self-report to malaria clinics in western Thailand, patients with mixed P. vivax–P. falciparum infections have higher fevers than patients with single-species infections, a distinction that cannot be attributed to differences in parasitemia. This observation warrants more detailed investigations, spanning wider ranges of ages and transmission environments.
Human malaria can be caused by any of 4 species of Plasmodium that occur in various geographically overlapping combinations in regions where they are endemic. Plasmodium falciparum is responsible for almost all mortality attributed to malaria, but Plasmodium vivax is the source of as much or more morbidity worldwide [1].
Where P. falciparum and P. vivax are both present, point-prevalence surveys usually report statistical deficits of mixed-species infections in the human population, relative to what would be expected if the species were independent [2, 3]. In a mixed P. vivax–P. falciparum infection, however, peaks of parasitemia typically alternate between species [4, 5]; PCR-based surveys [6, 7] and mathematical analyses [8] suggest that the statistical deficits reflect these individual-infection dynamics, because they are mediated by the detection thresholds of microscopy, and the results of classic longitudinal field studies [9–10] seem to be in line with this interpretation. One recent estimate is that mixed P. vivax–P. falciparum infections make up one-third to one-half of malaria infections in Thailand, rather than the reported 0.3%–0.7% [11].
A case series of adult inpatients in India showed higher frequencies of pernicious syndromes and drug resistance [12], and a review of hospital records in Malaysia found anemia to be more severe and cerebral malaria to be more frequent and more often fatal [13] in mixed P. vivax–P. falciparum infections, compared with single-species infections. Field studies in Sri Lanka [14] and Vanuatu [15], however, suggested that P. vivax ameliorates subsequent P. falciparum infections in children. A study in a refugee camp clinic in northwest Thailand reported lower frequencies of severe clinical outcomes, treatment failures, and anemia in mixed P. vivax–P. falciparum infections, compared with single-species P. falciparum infections [16–18]. Studies elsewhere in Thailand demonstrated that misdiagnoses of mixed-species infections as single-species infections can lead to the sudden emergence of 1 species as drug treatment clears the other [19], with sometimes fatal consequences when P. falciparum is the cryptic species [20].
Thus, to interpret trials of P. vivax vaccine candidates [21] and other population-level interventions, it would be helpful to know more about the effects of P. vivax on P. falciparum infections, and vice versa. For instance, several trials [22, 23] of the P. falciparum vaccine candidate Spf66 reported increases in the prevalence of P. vivax or the incidence of mixed P. vivax–P. falciparum infections. In this article, we report results of mixed P. vivax–P. falciparum infections in adults from a large, multiyear study in local outpatient malaria clinics in western Thailand.
METHODS
Data were collected during a study of malaria rapid diagnostic devices, the methods for which are fully described elsewhere [24–29]. Briefly, participants presented on their own initiative to existing outpatient malaria clinics operated by local public health authorities at Maesod (Tak Province, Thailand) during 28 May–28 August 1998 or 17 May–9 July 1999. They were ⩾15 years old, with fever (oral temperature, ⩾38°C), headache, or a self-reported history of fever within the previous 72 h. Severely ill patients were immediately referred to district hospitals and were not enrolled. During the study, clinic staff retained full responsibility for patient care; diagnostic and treatment decisions were made independent of the study protocol. The same criteria and protocol held in 2001, but the study was conducted 18 May–29 June, in the Mae Ku (Maesod) and Phob Phra Districts of Tak Province (Thailand), and patients ⩾20 years of age were enrolled. The protocol was reviewed by the Human Use Review Committee (Walter Reed Army Institute of Research), the Human Subjects Research Review Board (US Army Medical Research and Materiel Command), and the Ethical Review of Research Committee (Ministry of Public Health, Thailand).
At enrollment, patients were asked how long they had felt ill; responses were recorded as 1–8 days, with “8 days” representing any duration 17 days. They were also asked whether they had taken antimalarial drugs within the previous 2 weeks. Those who gave positive responses were not enrolled in 1998, but were enrolled and noted as such in 1999 and 2001. This analysis excludes 21 otherwise-eligible enrollees (none with mixed-species infections) who gave positive responses. Males comprised 66%–70% of all participants, 61%–63% of uninfected patients, 77%–81% of patients infected with P. falciparum, 70%–75% of patients infected with P. vivax, and 80%–92% of patients with mixed-species malaria. Log-linear analysis [30] shows a statistical interaction in each year between sex and malaria infection (P < .0001), but it also shows that male overrepresentation in the infected categories applies independently to each species and that the statistical interactions between P. falciparum and P. vivax infection are sex-independent. That is, the male bias among patients with single-species P. falciparum and P. vivax infections accounts for the male bias among patients with mixed-species infections. No patients <18 years old were enrolled in 1999. We removed the 83 enrollees <18 years old from the 1998 data (none of whom had mixed-species infections), rendering the 1998 and 1999 age distributions indistinguishable (P = .07, by the Mann-Whitney U test). The median age of participants was 25 years for each year, but the 2001 age distribution differed from the age distributions for 1998 and 1999 (P < .001), reflecting the absence of 18–19-year-old participants. Patients with mixed-species infections were not distinguishable from patients infected with P. falciparum or P. vivax on the basis of age (P > .41) or number of days reported as being ill (P > 0.13). Also, they were not distinguishable by calendar date of enrollment in 1998 or 1999 (P > .26), but in 2001, patients with mixed-species infection were enrolled earlier than patients with P. falciparum or P. vivax infection (median time of enrollment: patients with mixed-species infection, 2 June; patients with single-species infection, 9 or 11 June [P = .018 or P = .007]).
The oral temperature of each patient was taken immediately before blood was drawn. Approximately 2 μL of venous blood was drawn from each individual into an EDTA-filled tube for blood films and an automated blood count. Precise volumes of well-mixed whole blood were micropipetted and prepared as thick and thin smears on each of 3 precleaned slides by well-trained technicians following standardized procedures. One slide was used promptly by clinic staff for diagnosis and medical intervention, if such action was indicated. The remaining 2 slides were held overnight, then stained with 3% Giemsa. Each of 2 expert microscopists, blinded to the other’s interpretation, read the same one of these slides. Two hundred oil-immersion high power fields on the thick film were read before any blood smear was interpreted as showing negative results; the thin film was used only for species determination. Species identifications and density estimates were based solely on asexual blood forms. If, after 200 WBCs were counted, ⩾10 asexual parasites had been counted, the total number was recorded. If asexual parasites were present but numbered <10 per 200 WBCs, the microscopists continued examining the smear and counting parasites until 500 WBCs had been counted.
Blood tubes were promptly transported on ice to a field laboratory, where cell counts were performed using a Coulter automated cell counter (Beckman-Coulter). The extensive quality-assurance procedures employed are described elsewhere [24–29]. As expected, WBC counts had skewed distributions [30]. Detailed hematological data were collected from the patients enrolled in 2001 [27] but did not provide any basis for distinguishing patients with mixed-species infections from patients with P. falciparum or P. vivax infections (P >.085).
The 2308 malaria-infected and 2747 uninfected patients considered here include 1925 patients enrolled in 1998, 1118 patients enrolled in 1999, and 2012 patients enrolled in 2001; excluded are 109 patients, because microscopists disagreed regarding the presence or species of parasites. The ratio of P. falciparum–infected patients to P. vivax–infected patients was 1.02 in 1998, 0.97 in 1999, and 0.62 in 2001. The 36 cases for which 1 microscopist reported a mixed-species infection and the other microscopist reported a single-species infection were considered separately. The parasite densities used here are the averages of the 2 microscopists’ reports. We used the Mann-Whitney U test and Spearman’s rank correlation coefficient on distributions and the G-test on categorical data [31]. P values given are for 2-tailed tests.
RESULTS
The microscopists agreed on 15 mixed P. vivax–P. falciparum infections in 1998, 6 mixed infections in 1999, and 13 in 2001. In every case, P. falciparum parasitemia was higher than P. vivax parasitemia (table 1 and figure 1; P < .00001, by the Mann-Whitney U test); the median P. falciparum–to–P. vivax parasitemia ratio was 36 in 1998, 89 in 1999, and 316 in 2001. In 1998 and 2001, P. vivax parasitemia was lower among patients with mixed-species infections than among patients with single-species infections (P < .00001), but P. falciparum parasitemia among patients with mixed- and single-species infections was indistinguishable (P > .15). In 1999, P. vivax parasitemia among patients with mixed- and single-species infections was indistinguishable (P = .07), but P. falciparum parasitemia was higher among patients with mixed-species infections than among patients with single-species infections (P = .0055). In each year, the total parasitemia among patients with mixed-species infections was higher than the total parasitemia among patients with single-species P. vivax infections (P < .0005); in 1999, the total parasitemia among patients with mixed-species infections was higher than the total parasitemia among patients with single-species P. falciparum infections (P = .005), but in 1998 and 2001, parasitemias among patients with both types of infections were indistinguishable (P > .14).
Table 1.
Body temperature and asexual Plasmodium vivax and Plasmodium falciparum parasitemia in patients with single-species and mixed-species infections, by infection status.
| Infection status | No. of patients | Body temperature, median °C (95% Cl) | Parasitemia log10 parasites/μL (95% Cl), |
|---|---|---|---|
| Uninfected | 2747 | 37.4 (37.3–37.4) | … |
| Infeclted | |||
| P. vivax only | 1263 | 37.9 (37.7–38.0) | 3.51 (3.42–3.58) |
| P. falciparum only | 1011 | 38.3 (38.1–38.5) | 4.00 (3.92–4.09) |
| Mixed-species | 34 | 39.5 (38.4–40.0) | 2.27 (1.78–2.75)a |
| 4.32 (3.68–4.77)b | |||
P. vivax.
P. falciparum.
Figure 1.
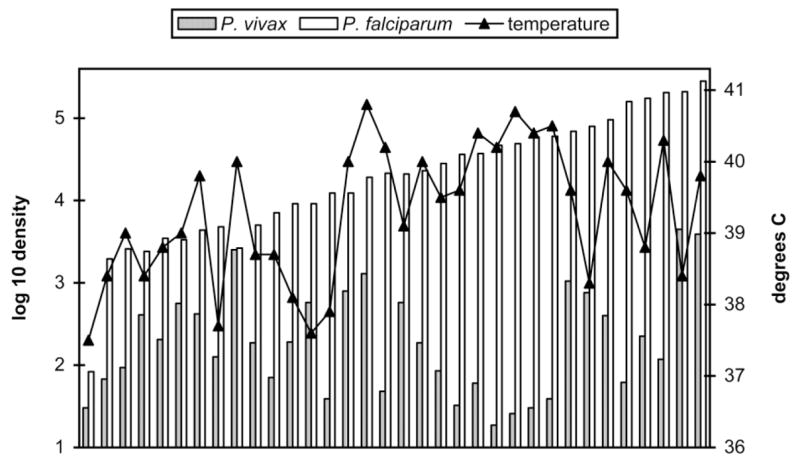
Per-μL densities of asexual Plasmodium vivax, Plasmodium falciparum, and body temperatures in 34 patients with mixed P. vivax–P. falciparum infections, by total asexual density (left to right).
Among patients with mixed-species infections, 12% of patient temperatures were <38°C and 24% of patient temperatures were >140°C. The comparable figures were 52% and 9%, respectively, for patients with single-species P. vivax infections and 40% and 12%, respectively, for patients with single-species P. falciparum infections (table 2). In each year, patient temperatures among patients with mixed-species infections were higher than those among uninfected patients (P < .00009, by the Mann-Whitney U test), P. vivax–infected patients (P < .002), or P. falciparum–infected patients (P = .029 in 1998, P = .009 in 1999, and P = .027 in 2001). In contrast to single-species infections [28], in the mixed-species infections, there was not a correlation between temperature and P. falciparum parasitemia (P > .35, by Spearman’s rank correlation coefficient), P. vivax parasitemia (P > .70), total parasitemia (P > .35), or the P. vivax–to–P. falciparum parasitemia ratio (P > .65).
Table 2.
Distribution of patient temperatures.
| Type of infection | <38°C | 38°C–40°C | 140°C |
|---|---|---|---|
| Plasmodium vivax only | 52 | 39 | 9 |
| Plasmodium falciparum only | 40 | 48 | 12 |
| Mixed-species | 12 | 64 | 24 |
NOTE. Data are % of patients.
Overall, the microscopists agreed on the presence or absence of gametocytes in 88% of patients in whom they had agreed on the presence and species of asexual parasites. Among these patients, the microscopists agreed that P. vivax gametocytes were present in 19% and P. falciparum gametocytes were present in 8% of patients with mixed-species infections, including 1 patient with gametocytes of both species. The comparable figures are that gametocytes were present in 57% of patients with P. vivax and 8% of patients with P. falciparum single-species infections; these few cases suggest a decreased incidence of P. vivax game-tocytemia but not of P. falciparum gametocytemia among patients with mixed P. vivax–P. falciparum infections [32].
WBC counts among patients with mixed-species infections varied widely, and in each year, WBC counts were intermediate between and statistically indistinguishable from WBC counts among patients with single-species infections with either P. vivax or P. falciparum (P > .25, by the Mann-Whitney U test). There was no correlation among patients with mixed-species infections between WBC count and P. vivax parasitemia, P. falciparum parasitemia, combined parasitemia, or patient temperature (P > .88, by Spearman’s rank correlation coefficient). In 29 of the 34 patients with mixed-species infections, the WBC count was <8000 cells/μL [28].
For an additional 36 patients, 1 microscopist reported a mixed-species and the other microscopist reported a single-species infection. Median temperature and total parasitemia were indistinguishable from those of the 34 patients for whom both microscopists reported a mixed-species infection (P > .17, by the Mann-Whitney U test). For 23 patients, the single species reported was P. falciparum, and among all 23 patients, the other microscopist reported that P. falciparum parasitemia was higher than P. vivax parasitemia. Among 8 of the 13 patients for whom the single infecting species reported was P. vivax, the other microscopist reported that P. falciparum parasitemia was higher than P. vivax parasitemia. Patient temperatures among patients in these various categories were indistinguishable (P >.55). If these 36 patients are added to the 34 patients agreed to have mixed-species infections, temperatures among infected patients were higher than those among uninfected (P <.0000001), P. vivax–infected (P < .0000001), or P. falciparum–infected patients (P = .00015). For 35 of these 36 patients, a third microscopist examined the slides, and for 5 patients the microscopist reported a mixed-species infection, for 15 patients the microscopist reported a P. vivax infection, and for 15 patients the microscopist reported a P. falciparum infection; the third microscopist disagreed with both initial microscopists regarding infections in 3 of 35 patients.
DISCUSSION
The statistical deficit of mixed-species infections reported here is in accord with most previous point-prevalence surveys from Asia [2, 3]. If the species were statistically independent, the expected number of mixed P. vivax–P. falciparum infections would be 268. Based on the “antagonism” between these species noted above [4, 5], one or the other species would be expected to dominate parasitemia at most points during a mixed infection. In each of the 34 patients with mixed-species infections reported here, P. falciparum parasitemia was much higher than P. vivax parasitemia. Patient temperatures were higher in patients with mixed P. vivax–P. falciparum infections than in patients with single-species infections. However, in contrast to patients with single-species infections [28], among patients with mixed-species infections, there was no correlation between patient temperature and parasitemia, for either or both species. Despite the complexity of fever curves, synchronization, and (P. falciparum) sequestration in malaria infections, the distinctions were seen at presentation. These are striking results, but they call for cautious interpretation.
First, reports that include data regarding frequencies of mixed-species infections seldom include data regarding the species densities of those infections, so it is possible that, in this regard, our results are not as unusual as they appear to be. In the only data of which we are aware, from a clinic in rural Pakistan, P. vivax parasitemia was higher than P. falciparum parasitemia in 14% and similar in 38% of patients with mixed P. vivax–P. falciparum infections. That study showed no statistical deficit of mixed-species infections [33] and a similar prevalence of mixed-species infections among patients younger and older than 13 years [34].
Second, despite our extraordinarily rigorous procedures, it is almost certain that some mixed-species infections went undetected. Detection is generally an inverse function of parasite densities, and with microscopy, the fields examined are a sample of uneven distributions of parasites across blood films [26, 29]. It is not clear to us, for instance, how to interpret the 36 cases in which only 1 of the microscopists reported a mixed-species infection, but a similar study supplemented by quantitative PCR detection techniques could be informative, perhaps even with respect to whether our seemingly robust results regarding patient temperatures indicate some potential diagnostic value in raising or lowering suspicion of a cryptic mixed-species infection.
Third, it is not clear whether higher fevers, per se, indicate greater clinical severity or more-effective immune responses, hence, whether our results conflict with those that suggest mixed-species infections ameliorate clinical malaria. As noted above, for instance, we found no evidence that the frequency of anemia differed between patients with mixed-species infections and patients with single-species infections in 2001. It is possible that in mixed P. vivax–P. falciparum infections in adults, fever does not directly correspond to parasite density but is high when P. falciparum parasitemia is greater than P. vivax parasitemia and low when P. vivax parasitemia is greater than P. falciparum parasitemia. If adults with lower temperatures are less likely to self-report to clinics, a study based on passive case detection would be biased accordingly.
Effective immune responses against P. vivax are thought to develop after fewer infections than those against P. falciparum [35–37], hence, all else being equal, at younger ages. Parasitemia “thresholds” for fever are thought to be lower with P. vivax than with P. falciparum [38–39], although with P. falciparum, parasitemia thresholds may decrease with age [37]. Thus, mixed-species infections may appear more often in younger age groups, perhaps with higher densities of P. vivax and different patterns or intensities of fever. Parasitemia and fever in P. vivax, P. falciparum, and mixed-species infections are likely to peak—and ameliorative effects are likely to be most apparent—at different ages. For instance, although the refugee camp clinic study cited above [16–18] did not report patient temperatures, parasitemia levels, or age-specific protective effects for patients with mixed-species infections, in the camp population, the incidence of P. vivax infection peaked during ages 0–4 years, the incidence of P. falciparum infection peaked during ages 20–29 years, and the incidence of mixed-species infection peaked during ages 4–15 years [38].
Furthermore, given that P. falciparum and P. vivax typically co-occur in regions with seasonal transmission, in which their prevalences peak at different points in the year, we note that in some previous studies, cases of P. falciparum infection greatly outnumbered cases of P. vivax infection [15, 33, 36]. In the Thai refugee camp clinic [16], and in our 1998 and 1999 study populations, the species frequencies were nearly identical, although cases of P. vivax infection outnumbered cases of P. falciparum infection in our 2001 data (in accordance with national trends). A mixed-species infection in a human can result from a single bite by a mosquito infected with multiple species or from multiple bites by mosquitoes infected with single species. The relative frequency of these events depends on the age distribution in the vector population, which changes during a season and year [40]; the order in which the Plasmodium species infect may be critical to their dynamics in the human [8]. Thus, the reported frequencies of mixed-species infections are likely to vary by season and by the particular time during a season at which a study occurs. Though we found no association between the slightly earlier calendar dates of enrollment each year (median enrollment times: 14 July in 1998, 22 June in 1999, and 11 June in 2001), the increase in relative P. falciparum parasitemia among patients with mixed-species infections, the decrease in relative P. falciparum frequency overall, or changes in other variables, we recognize that calendar dates do not necessarily reflect critical environmental and entomological variables.
We expect that critical distinctions between the species, with respect to stimulating fever, in single- and mixed-species infections, in relation to parasite densities, can be discovered through careful studies that integrate molecular, clinical, and field studies of malaria parasites, hosts, and vectors. Most helpful would be studies that encompass wider age spans and >1 time point during each infection, coordinated with active detection of asymptomatic infections in catchment areas.
In closing, we note that results based even on highly expert microscopy may differ by an order of magnitude from those based on PCR [11], a gap similar to that in our data between the number of mixed-species infections observed and the number expected on the basis of statistical independence between the species. Clearly, if the true number of mixed-species infections was close to the expected number and if the distribution of temperatures was close to the distribution of temperatures observed, our major conclusion here would be even more striking. We previously suggested that frequencies of detection represent an intersection of biological phenomena and methodological shortcomings [2]. If biological interactions between species are such that one may substantially exacerbate or ameliorate the effects of another, it is imperative that the technical obstacles that block our comprehension of the biology be addressed and overcome.
Acknowledgments
We thank the Armed Forces Research Institute of Medical Sciences field study team for their dedication and technical assistance, and we gratefully acknowledge the contributions of J. Chupasko, D. Hodermarsky, A. Kiszewski, D.P. Mason, M.F. McKenzie, and 2 anonymous reviewers.
Financial support. US Army Medical Research and Materiel Command.
Footnotes
The views presented in this article are those of the authors and do not represent those of the authors’ respective institutions.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.White NJ, Breman JG. Malaria and babesiosis. In: Braunwald E, Fauci AS, Isselbacher KJ, et al., editors. Harrison’s principles of internal medicine. New York: McGraw-Hill; 2001. pp. 1203–13. [Google Scholar]
- 2.McKenzie FE, Bossert WH. Mixed-species Plasmodium infections of humans. J Parasitol. 1997;83:593–600. [PMC free article] [PubMed] [Google Scholar]
- 3.McKenzie FE, Bossert WH. Multispecies Plasmodium infections of humans. J Parasitol. 1999;85:12–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd MF, Kitchen SF. Simultaneous inoculation with Plasmodium vivax and Plasmodium falciparum. Am J Trop Med. 1937;17:855–61. [Google Scholar]
- 5.Boyd MF, Kitchen SF, Matthews CB. Consecutive inoculations with Plasmodium vivax and Plasmodium falciparum. Am J Trop Med. 1938;18:141–50. [Google Scholar]
- 6.Brown AE, Kain KC, Pipithkul J, Webster HK. Demonstration by the polymerase chain reaction of mixed Plasmodium falciparum and P. vivax infections undetected by conventional microscopy. Trans R Soc Trop Med Hyg. 1992;86:609–12. doi: 10.1016/0035-9203(92)90147-5. [DOI] [PubMed] [Google Scholar]
- 7.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–92. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 8.Mason DP, McKenzie FE. Blood-stage dynamics and clinical implications of mixed Plasmodium vivax–Plasmodium falciparum infections. Am J Trop Med Hyg. 1999;61:367–74. doi: 10.4269/ajtmh.1999.61.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earle WC, Perez M, Del Rio J, Arzola C. Observations on the course of naturally acquired malaria in Puerto Rico. Puerto Rican J Pub Health Trop Med. 1939;14:391–406. [Google Scholar]
- 10.Hill RB, Cambournac FJC, Simoes MP. Observations on the course of malaria in children in an endemic region. Am J Trop Med Hyg. 1943;23:147–62. [Google Scholar]
- 11.Snounou G, White NJ. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol. 2004;20:333–9. doi: 10.1016/j.pt.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Gopinathan VP, Subramanian AR. Vivax and falciparum malaria seen at an Indian service hospital. J Trop Med Hyg. 1986;89:51–5. [PubMed] [Google Scholar]
- 13.Lyn PC. Cerebral malaria and mixed falciparum-vivax infections. Ann Acad Med Singapore. 1987;16:310–2. [PubMed] [Google Scholar]
- 14.Gunewardena DM, Carter R, Mendis KN. Patterns of acquired anti-malarial immunity in Sri Lanka. Mem Inst Oswaldo Cruz. 1994;89:63–5. doi: 10.1590/s0074-02761994000600015. [DOI] [PubMed] [Google Scholar]
- 15.Maitland K, Williams TN, Bennett S, et al. The interaction between Plasmodium falciparum and P. vivax in children on Espiritu Santo island, Vanuatu. Trans Roy Soc Trop Med Hyg. 1996;90:614–20. doi: 10.1016/s0035-9203(96)90406-x. [DOI] [PubMed] [Google Scholar]
- 16.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–62. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 17.Price RN, Nosten F, Luxemburger C, et al. Artesunate/mefloquinetreatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:574–7. doi: 10.1016/s0035-9203(97)90032-8. [DOI] [PubMed] [Google Scholar]
- 18.Price RN, Simpson JA, Nosten F, et al. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65:614–22. doi: 10.4269/ajtmh.2001.65.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looareesuwan S, White NJ, Chittamas S, Bunnag D, Harinasuta T. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet. 1987;2(8567):1052–5. doi: 10.1016/s0140-6736(87)91479-6. [DOI] [PubMed] [Google Scholar]
- 20.Mason DP, Krudsood S, Wilairatana P, et al. Can treatment of P. vivax lead to a unexpected appearance of falciparum malaria? Southeast Asian J Trop Med Public Health. 2001;32:57–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Arevalo-Herrera M, Herrera S. Plasmodium vivax malaria vaccine development. Mol Immunol. 2001;38:443–55. doi: 10.1016/s0161-5890(01)00080-3. [DOI] [PubMed] [Google Scholar]
- 22.Noya OG, Berti YG, de Noya BA, et al. A population-based clinical trial with the SPf synthetic Plasmodium falciparum malaria vaccine in Venezuela. J Infect Dis. 1994;170:396–402. doi: 10.1093/infdis/170.2.396. [DOI] [PubMed] [Google Scholar]
- 23.Nosten F, Luxemburger C, Kyle DE, et al. Phase I trial of the SPf66 malaria vaccine in a malaria-experienced population in Southeast Asia. Am J Trop Med Hyg. 1997;56:526–32. doi: 10.4269/ajtmh.1997.56.526. [DOI] [PubMed] [Google Scholar]
- 24.Forney JR, Magill AJ, Wongsrichanalai C, et al. Malaria rapid diagnostic devices: performance characteristics of the ParaSight F device determined in a multisite field study. J Clin Microbiol. 2001;39:2884–90. doi: 10.1128/JCM.39.8.2884-2890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forney JR, Wongsrichanalai C, Magill AJ, et al. Devices for rapid diagnosis of malaria: evaluation of prototype assays that detect Plasmodium falciparum Histidine-Rich Protein 2 and a Plasmodium vivax–specific antigen. J Clin Microbiol. 2003;41:2358–66. doi: 10.1128/JCM.41.6.2358-2366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenzie FE, Sirichaisinthop J, Miller RS, Gasser RA, Jr, Wongsri-chanalai C. Dependence of malaria detection and species diagnosis by microscopy on parasite density. Am J Trop Med Hyg. 2003;69:372–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Erhart LM, Yingyuen K, Chuanak N, et al. Hematologic and clinical indices of malaria in a semi-immune population of western Thailand. Am J Trop Med Hyg. 2004;70:8–14. [PubMed] [Google Scholar]
- 28.McKenzie FE, Prudhomme WA, Magill AJ, et al. White blood cell counts and malaria. J Infect Dis. 2005;192:323–30. doi: 10.1086/431152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Meara WP, McKenzie FE, Magill AJ, et al. Sources of variability in determining malaria parasite density by microscopy. Am J Trop Med Hyg. 2005;72:593–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Bain BJ. Blood cells. Oxford: Blackwell Science; 2002. [Google Scholar]
- 31.Sokal RR, Rohlf FJ. Biometry. New York: WH Freeman; 1981. [Google Scholar]
- 32.Price RN, Nosten F, Simpson JA, et al. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60:1019–23. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 33.Fox E, Strickland GT. The interrelationship of Plasmodium falciparum and P. vivax in the Punjab. Trans Roy Soc Trop Med Hyg. 1989;83:471–3. doi: 10.1016/0035-9203(89)90251-4. [DOI] [PubMed] [Google Scholar]
- 34.Strickland GT, Fox E, Hadi H. Malaria and splenomegaly in the Punjab. Trans Roy Soc Trop Med Hyg. 1988;82:667–70. doi: 10.1016/0035-9203(88)90188-5. [DOI] [PubMed] [Google Scholar]
- 35.Molineaux L. The epidemiology of human malaria as an explanation of its distribution, including some implications for its control. In: Wernsdorfer WH, McGregor I, editors. Malaria. Edinburgh: Churchill Livingstone; 1988. pp. 913–88. [Google Scholar]
- 36.Rosenberg R, Andre RG, Ngampatom S, Hatz C, Burge R. A stable, oligosymptomatic malaria focus in Thailand. Trans R Soc Trop Med Hyg. 1990;84:14–21. doi: 10.1016/0035-9203(90)90366-m. [DOI] [PubMed] [Google Scholar]
- 37.Prybylski D, Khaliq A, Fox E, Sarwari AR, Strickland GT. Parasite density and malaria morbidity in the Pakistani Punjab. Am J Trop Med Hyg. 1999;61:791–801. doi: 10.4269/ajtmh.1999.61.791. [DOI] [PubMed] [Google Scholar]
- 38.Luxemburger C, Thwai KL, White NJ, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans Roy Soc Trop Med Hyg. 1996;90:105–11. doi: 10.1016/s0035-9203(96)90102-9. [DOI] [PubMed] [Google Scholar]
- 39.McKenzie FE, Jeffery GM, Collins WE. Plasmodium vivax blood-stage dynamics. J Parasitol. 2002;88:521–35. doi: 10.1645/0022-3395(2002)088[0521:PVBSD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKenzie FE, Bossert WH. Mixed-species Plasmodium infections of Anopheles. J Med Entomol. 1997;34:417–25. doi: 10.1093/jmedent/34.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]


