Abstract
Inward-rectifier K+ channels of the ROMK (Kir1.1) subtype are responsible for K+ secretion and control of NaCl absorption in the kidney. A hallmark of these channels is their gating by intracellular pH in the neutral range. Here we show that a lysine residue close to TM1, identified previously as a structural element required for pH-induced gating, is protonated at neutral pH and that this protonation drives pH gating in ROMK and other Kir channels. Such anomalous titration of this lysine residue (Lys-80 in Kir1.1) is accomplished by the tertiary structure of the Kir protein: two arginines in the distant N and C termini of the same subunit (Arg-41 and Arg-311 in Kir1.1) are located in close spatial proximity to the lysine allowing for electrostatic interactions that shift its pKa into the neutral pH range. Structural disturbance of this triad as a result from a number of point mutations found in patients with antenatal Bartter syndrome shifts the pKa of the lysine residue off the neutral pH range and results in channels permanently inactivated under physiological conditions. Thus, the results provide molecular understanding for normal pH gating of Kir channels as well as for the channel defects found in patients with antenatal Bartter syndrome.
Inwardly rectifying K+ channels in the apical membrane of the thick ascending limb of Henle (TAL), the distal convoluted tubule (DCT), and the cortical collecting duct (CCD) play a key role in the transport of K+ ions across the tubular epithelium (1). This K+ conductance is responsible for K+ secretion and, by recycling K+ into the lumen, for control of NaCl absorption via the Na-K-2Cl-cotransporter (TAL) and the epithelial Na+ channel (DCT and CCD) (2, 3).
ROMK was the first inward-rectifier to be cloned (4) and demonstrates the prototypic transmembrane topology of this class of K+ channels, comprising hydrophilic N and C termini and two hydrophobic segments (TM1 and TM2) flanking a well-conserved pore domain (Kir channels, for review see refs. 5 and 6). Kir channels are assembled from four subunits (7–9) and form homo- and heteromultimeric channel proteins (7, 10). Common to all Kir channels is their inwardly rectifying current-voltage relation (I–V), which may be weak or strong and is predominantly caused by a voltage-dependent block of the channel pore by intracellular spermine (SPM, refs. 11–13). ROMK (Kir1.1) exists in three N-terminal splice variations, ROMK1–3, with ROMK2 shortened by 19 aa and ROMK3 exhibiting an extension of seven residues with respect to ROMK1 (14, 15). These splice variant mRNAs are differentially expressed in subpopulations of renal tubular cells (14, 16). All ROMK variants are weakly rectifying K+ channels, and similar to their native counterparts, cloned ROMK channels are gated by intracellular pH (pHi) with acidification leading to channel closure (1, 17–19). An intracellular lysine that resides close to TM1 (Lys-80, preM1 site), common to all forms of ROMK, has been identified as the structural element necessary for pH-dependent gating (18). In addition, structural rearrangements within the hydrophilic intracellular domains have been shown to occur during gating transitions (20). Thus, Cys-49 in the N terminus and Cys-308 in the C terminus displayed state-dependent accessibility to sulfhydryl reagents with modification only occurring in the pH-inactivated state (20). Examination of the single-channel behavior demonstrated that the closed time increased on acidification, whereas the unitary conductance remained unchanged (18, 21).
Recently, the ROMK gene (KCNJ1) as well as the gene encoding the Na-K-2Cl cotransporter (NKCC2) have been shown to coseggregate with antenatal Bartter syndrome (aBS) (22–24). This syndrome, also termed hyperprostaglandine E syndrome, is a hereditary tubulopathy with an incidence estimated to 1 in 50,000–100,000 newborns (25). The mode of inheritance is autosomal recessive. In the fetus, the disease causes excessive saluresis and polyuria, leading to polyhydramnion and preterm delivery (25). Postnatally, affected infants present with a typical pattern of impaired tubular reabsorption in the thick ascending limb of Henle including salt wasting, polyuria, and hypercalciuria with subsequent nephrocalcinosis. Characteristically, both renal and systemic formation of prostaglandine E2 are markedly stimulated, resulting in further aggravation of saluretic polyuria, secretory diarrhea, and failure to thrive (25). Analysis of the ROMK gene in individual aBS patients identified a number of point mutations in the core region as well as in the intracellular N and C termini (22, 24, 26, 27). Some of these mutations recently were shown to result in a loss of Kir1.1 channel function in heterologous expression experiments, although the mechanisms leading to nonfunctional channels are largely unknown (27, 28). A loss of ROMK function will result in impaired renal K+ secretion and NaCl reabsorption and lead to the clinical phenotype of aBS.
Here we investigate the molecular mechanism underlying pH gating of ROMK channels in the physiological pH range and test its relevance for the defective function of these channels in patients with aBS.
Materials and Methods
Mutagenesis and cRNA Synthesis.
Site-directed mutagenesis was performed as described (11) and verified by sequencing. For heterologous expression, all constructs were subcloned into the pBF expression vector (B.F., unpublished work); capped mRNAs for wild-type and mutant subunits of Kir1.1, Kir4.1, Kir6.2, and SUR1 were synthesized in vitro by using the mMESSAGE mMACHINE kit (Ambion, Austin, TX). For the coexpression experiments in Fig. 4, the ratio of Kir1.1(K80M):Kir1.1(R311Q, N171D) mRNAs was varied from 1:0.1 to 1:100 with the amount of mRNA coding for Kir1.1(K80M) kept constant.
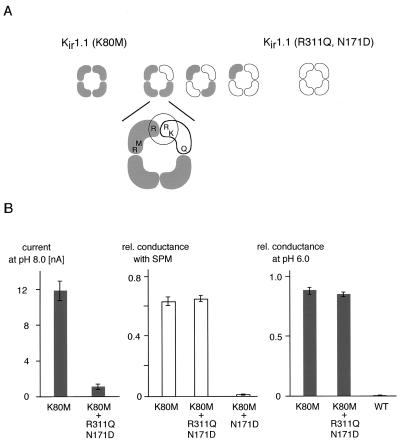
Figure 4.
Arg-Lys-Arg triad and gating is realized within one subunit. (A) (Upper) Cartoon illustrating the coexpression experiment (see text) designed to test for formation of the Arg-Lys-Arg triad via intersubunit interaction between Arg-41, Lys-80 [in Kir1.1(R311Q, N171D)], and Arg-311 [in Kir1.1(K80M)]. (Lower) Intersubunit interaction in a heteromultimeric complex assembled from three Kir1.1(K80M) subunits and one Kir1.1(R311Q, N171D) subunit. (B) Histograms illustrating current amplitudes (at −80 mV; Left), relative conductance at pH 8.4 in the presence of 100 μM SPM (at 50 mV; Center), and relative conductance at pH 6.0 in the absence of SPM (Right) observed with homomeric Kir1.1(K80M) and channels forming on coexpression of Kir1.1(K80M) and Kir1.1(R311Q, N171D); all values are mean ± SD of 16–21 experiments (for the amplitudes) or of 8–11 experiments (for the conductances); relative conductance is normalization of conductance with respect to that observed in the absence of SPM or at a pHi of 9.6. All coexpression experiments were done with an mRNA ratio of 1:10 (K80M: R311Q,N171D or K80M:N171D); note that all channels formed in the K80M:N171D coexpression were blocked by 100 μM SPM at 50 mV.
Electrophysiology.
Xenopus oocytes were prepared and injected as described (11). Giant patch recordings in inside-out configuration were made at room temperature (≈23°C) 3–7 days after injection. Pipettes used were made from thick-wall borosilicate glass, had resistances of 0.3–0.6 MΩ (tip diameter of 20–30 μm), and were filled with 120 mM KCl, 10 mM Hepes, and 1.8 mM CaCl2 (pH adjusted to 7.2 with KOH). Currents recorded in response to voltage steps or ramps were sampled at 1 kHz with an EPC9 amplifier (HEKA Electronics, Lamprecht, Germany), with analog filter set to 3 kHz (−3 dB). Intracellular solution (K-Int) was applied to excised inside-out patches via a multibarrel pipette as described (11) and had the following composition: 100 mM KCl, 10 mM Hepes, 10 mM K2EGTA, 0.1 mM DTT; pH was adjusted to 8.0 with KOH and titrated to the given pH with HCl or KOH. The pH used in inside-out experiments ranged from 4.0 to 10.2; pHis above or below this range led to an almost immediate breakdown of the patches.
9-Fluorenylmethoxycarbonyl-chloride (FmocCl) was set up in water-free dioxane as a 300 mM stock and added to the modification buffer (100 mM KOH, 10 mM KH2PO4, pH adjusted to 7.5 with HCl) before each experiment.
Current-pHi relations were obtained and evaluated as described (18); formation of heteromultimeric channel populations was tested as given in ref. 7. Briefly, I–V relations obtained from ramp recordings (in the absence and presence of SPM) were transferred to conductance-voltage plots and fitted with a single or multiple Boltzmann functions. Component(s) with an SPM block different from Kir1.1(K80M) and contributing to the total block by ≥5% were not detected in any of these experiments.
Computational work was done on a Macintosh G3 using commercial software (igor, WaveMetrics, Lake Oswego, OR) for fitting.
Immunocytochemistry.
After removal of the vitelline membrane, oocytes were fixed in Dent's fixative overnight at −20°C (29). Oocytes were incubated at room temperature with the M2 anti-Flag antibody (Sigma, 3 hr) and subsequently with a Cy3-conjugated secondary antibody (Dianova, Hamburg, Germany, dilution 1:200, 1 hr). Stained oocytes were postfixed with 3.7% paraformaldehyde, embedded with Technovit 7100 (Heraeus), and cut to 4-μm sections. Analysis was performed with a fluorescence microscope (Axioskop, Zeiss).
Mutation Analysis and Clinical Course of aBS Patients with Triad Mutations.
Genomic DNA was extracted from peripheral leukocytes. Aberrant band patterns for the KCNJ1 gene were sought by single-strand conformation polymorphism analysis as described (24). A standard 30-cycle PCR was performed with 50 ng of genomic DNA, and the PCR products were sequenced by using 5′ cy5-labeled primers on an ALF express sequencing system (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Analysis of 19 aBS patients revealed two unrelated individuals with mutations of R311; both patients were heterozygous with patient 1 displaying exchanges R311Q and F325C, and patient 2 harboring mutations R311W and L220F. Both patients displayed a clinical course indicative of defective ROMK function including marked polyhydramnion with premature birth, marked hyperkalemia in the postnatal period and hypokalemia later on, severe salt wasting, and hyperreninemic hyperaldosteronism.
Results
pH Gating in Kir Channels Is Driven by Protonation of a Lysine Residue with Anomalous Titration.
The pH-dependent gating of ROMK1 channels as recorded in giant inside-out patches from Xenopus oocytes is illustrated in Fig. 1A. Intracellular acidification leads to channel closure whereas alkalinization results in channel activation. Under steady-state conditions, pH gating showed half-maximal activation (pH0.5) at pHi 6.8 and exhibited a Hill coefficient of around 3, indicating cooperativity of this process (not shown).
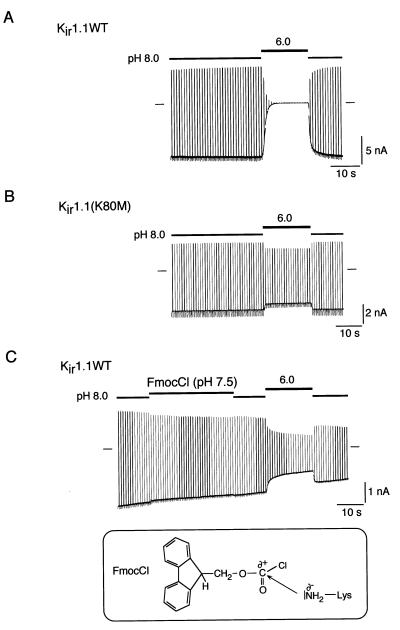
Figure 1.
pH gating of Kir1.1 channels is driven by protonation of Lys-80 in the neutral pH range. (A) pH gating of Kir1.1 channels measured in a giant inside-out patch from Xenopus oocytes on switching pHi from 8.0 to 6.0 (inactivation) and from 6.0 to 8.0 (recovery from inactivation). Currents were recorded at a membrane potential of −80 mV, which was stepped up to 50 mV for 50 ms every 0.9 s. Solution application and changes in pHi are illustrated by horizontal bars, time and current scales as indicated. (B) Same experiment as in A but with the pH-insensitive mutant Kir1.1(K80M) in which Lys-80 was replaced by methionine. (C) (Upper) pH gating is abolished in Kir1.1 channels by application of FmocCl at pHi 7.5 before acidification. Changes in pHi and application of FmocCl as indicated by horizontal bars. (Lower) Structure of FmocCl and its chemical reaction (nucleophilic substitution) with the amino group of a lysine residue.
We previously had hypothesized that pH gating is triggered by protonation of Lys-80, necessitating a shift in pKa of the amino group of this residue by more than 3 pH units compared with its nominal value of 10.5 (18). To directly test for this hypothesis, the protonation state of Lys-80 was investigated by application of FmocCl, a reagent that reacts with NH2 groups but not with protonated NH3+ groups (Fig. 1C Lower; ref. 30). As a result of this reaction, a urethane is formed with the amino group of Lys-80, which then is no longer a target for protonation. Fig. 1C shows that ROMK1 channels treated with FmocCl at pHi 7.5 before acidification no longer exhibited pH-dependent gating and closely resembled a mutant channel in which Lys-80 had been replaced by a nontitratable methionine [Kir1.1(K80M); Fig. 1B]. This result indicated that the side chain of Lys-80 is deprotonated at pHi 7.5 and that protonation of the amino group is required for pH gating of ROMK channels.
Analogous pH gating driven by protonation of a lysine residue in the neutral pH range was observed in Kir4.1 wild type (31), as well as in mutants of Kir2.1 (18) and Kir6.2, where lysine has been introduced at the position homologous to 80 in Kir1.1 (see also Discussion). This finding suggested a common structural basis and raised the question about the molecular determinants that endow this lysine residue with anomalous titration within Kir proteins.
Anomalous Titration of Lys-80 Is the Result of an Arg-Lys-Arg Triad.
Based on the observations that the side chain of Lys-80 is accessible from the cytoplasm (Fig. 1 A and C), and that the concentration of hydrogen ions required for its protonation was more than 103 times the nominal value, we hypothesized that in the context of the Kir1.1 protein Lys-80 might be shielded by a positively charged environment repelling hydrogen ions from its amino group. Accordingly, neutralization of such an environment should shift titration of Lys-80 back to its nominal value and thus result in channels permanently inactivated in the neutral pH range.
To test this hypothesis, all intracellular arginine (Arg) and lysine residues conserved among the known Kir subtypes (Fig. 2A) were individually replaced by glutamine (Gln) and the resulting channels were tested for pH gating. As summarized in Fig. 2A, most of the Lys/Arg-Gln exchanges resulted in channels with pH gating either identical to wild type or with only a minor shift in the respective pH0.5. However, for charge neutralizations at position 41 (R41Q) in the N terminus and at position 311 in the C terminus (R311Q) no channel activity was detected up to a pHi of 10.0 (Fig. 2B; 21 patches from five oocytes), although these proteins were incorporated into the plasma membrane as shown by immunostaining [Fig. 2C; data for Kir1.1(R41Q) not shown]. If the loss of channel activity was attributable to pH inactivation, an exchange of Lys-80 in the R311Q or R41Q protein by a nontitratable methionine [Kir1.1(R41Q, K80M), Kir1.1(R311Q, K80M)] should rescue channel function independent of pH. This rescue was indeed observed for both Arg-Gln mutations as well as for the double Arg-Gln exchange R41,311Q [Kir1.1(R41, 311Q, K80M)], indicating that the combined effects of arginines 41 and 311 endow the anomalous titration of Lys-80 in the Kir1.1 protein (Fig. 2D). The significance of the C-terminal arginine in Kir proteins was demonstrated by the homologous mutation in Kir4.1 [Kir4.1(R294Q)]. In these channels, pH-dependent gating could be monitored in excised patches and displayed a pH0.5 that was shifted by more than 3 pH units with respect to Kir4.1 wild type (Fig. 3 A and B). Thus, neutralization of the C-terminal arginine did not affect the ability of the channel to open and close but shifted the pH dependence of gating. The difference in pKa of the lysine residues in Kir1.1 and Kir4.1 may be caused by different contributions of the N-terminal arginine or by other structural variations between the two Kir proteins.
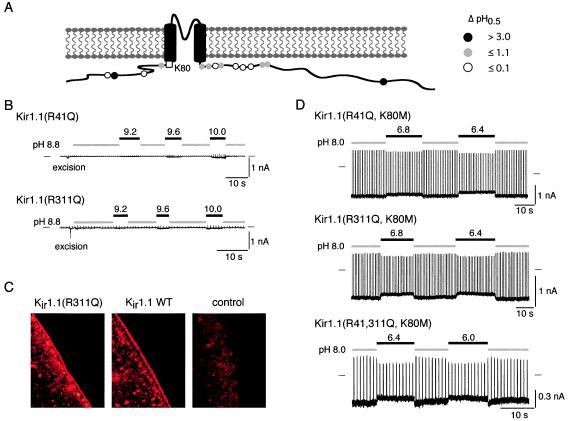
Figure 2.
Neutralization of Arg-41 and Arg-311 shifts titration of Lys-80 to the alkaline pH range by several pH units. (A) Cartoon summarizing the shift in pH0.5 (ΔpH0.5) observed on neutralization of the Arg/Lys residues in the N and C termini conserved among all Kir proteins. Note that only R41Q and R311Q led to shifts in pH0.5 (ΔpH0.5) >3 pH units. (B) Patch recordings from oocytes expressing Kir1.1(R41Q) (Upper) and Kir1.1(R311Q) (Lower) at the pHis indicated. Protocol as in Fig. 1. (C) Immunostaining of FLAG-tagged Kir1.1(R311Q) and Kir1.1 wild-type channels. Note staining of the plasma membrane in oocytes expressing either the Kir1.1 wild-type or mutant subunit, whereas no staining was obtained in a control oocyte (water injected). (D) Rescue of channel activity by replacement of Lys-80 with methionine in R41Q, R311Q, and R41,311Q channels. Recordings as in A from Kir1.1(R41Q,K80M), Kir1.1(R311Q,K80M), and Kir1.1(R41, 311Q,K80M) at the pHis indicated.
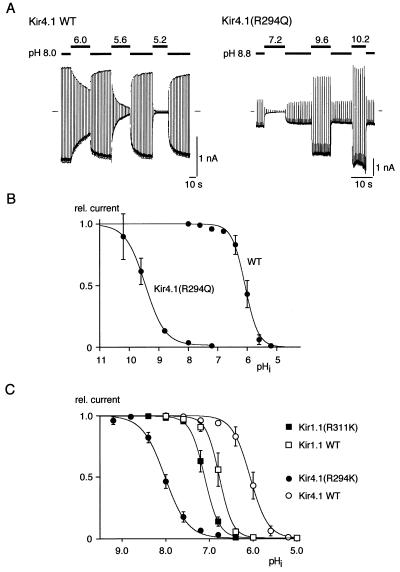
Figure 3.
Sensitivity of pH gating to side-chain variations at the C-terminal residue in Kir4.1 and Kir1.1 channels. (A and B) Shift in pH gating induced by neutralization of the C-terminal residue in Kir4.1 channels. (A) Recordings from Kir4.1 wild-type and Kir4.1(R294Q) channels at the pHis indicated; experimental protocol as in Fig. 1. (B) Current-pHi relation of Kir4.1(R294Q) and Kir4.1 wild type. Note the shift in pH0.5 observed for the mutant channels with respect to wild type [pH0.5 and Hill coefficient were 9.5 and 1.4 for Kir4.1(R294Q) and 6.1 and 2.1 for Kir4.1 wild type]. (C) Effect of an Arg-Lys exchange at the C-terminal site in Kir1.1 and Kir4.1 channels on pH gating. Current-pHi relation obtained from Kir1.1 wild type and Kir1.1(R311K) (squares) and from Kir4.1 wild type and Kir4.1(R294K) (circles). Lines represent fit of a Boltzmann function to the data (mean ± SD of eight and seven experiments for R311K and R294K, respectively). pH0.5 and Hill coefficient, respectively, were 7.1 and 2.7 for Kir1.1(R311K) and 8.0 and 1.7 for Kir4.1(R294K).
Next we tested the requirement of a positively charged side chain at the identified sites in the N and C termini by Arg-Lys exchanges in Kir1.1 [Kir1.1(R41K), Kir1.1(R311K)] and Kir4.1 [Kir1.1(R294K)]. Fig. 3C shows that in Kir1.1(R311K) pH gating was shifted to more alkaline pH values by about 0.3 pH units, whereas in Kir4.1(R294K) the shift was almost 2 pH units. For Kir1.1(R41K), no channel activity was observed up to a pHi of 10.0 (19 patches from six oocytes; not shown). These results indicated that a positive charge at either determinant is necessary but not sufficient to accomplish lysine titration as in wild-type channels. In addition, because the mutagenesis effects shown in Fig. 3C were likely caused by exchange of a guanidino group for an aminomethylene group, these results strongly suggest close spatial proximity of the lysine and the two identified arginine residues within the Kir proteins.
The Arg-Lys-Arg Triad Is Formed Within Individual Subunits.
The results presented above do not distinguish whether the interactions between Lys-80 and arginines 41 and 311 occur within the same (intrasubunit) or different subunits (intersubunit) of the tetrameric channel complex. To address this question, the non-pH-gated (i.e., constitutively open) Kir1.1(K80M) and the pH-inactivated Kir1.1(R311Q) subunit were coexpressed. Kir1.1(R311Q) was additionally “tagged” by the aspartate (D) in M2 (N171D), which is responsible for strong voltage-dependent block of the pore by SPM (32) and allows detection of the Kir1.1(R311Q,N171D) subunit(s) in a heteromeric channel population (7).
If the interaction between R41, K80, and R311 occurred between two subunits via intersubunit interaction, then at least heteromeric channels assembled from three Kir1.1(K80M) and one Kir1.1(R311Q,N171D) subunit (highlighted in Fig. 4A) should be (i) gated by pH because of reconstitution of the Arg-Lys-Arg triad and (ii) sensitive to block by SPM because of integration of D171 into the channel tetramer (7).
As shown in Fig. 4B (Left and Center), coexpression of Kir1.1(R311Q, N171D) to the Kir1.1(K80M) subunit resulted in a large reduction of the current amplitude; the residual current did not contain any component ≥5% that was blocked by SPM in a strongly voltage-dependent manner (tested in the pH range from 6.0 to 9.2; see also Materials and Methods). This result suggested that heteromeric channels were formed but did not conduct current (in the pH range from 6.0 to 9.2). Accordingly, the residual currents were indistinguishable from homomeric Kir1.1(K80M) channels, i.e., they did not show pH-induced inactivation (Fig. 4B Right) and exhibited only weakly voltage-dependent block by SPM (Fig. 4B Center). Very similar results as described for the Kir1.1(K80M)/Kir1.1(R311Q,N171D) coexpression were obtained when Kir1.1(R41Q)/Kir1.1(R41Q,N171D) was coexpressed with Kir1.1(K80M) (data not shown).
The result of heteromeric channels being nonfunctional indicated that Lys-80 interacted with Arg-41 and Arg-311 resident in the same subunit and ruled out intersubunit interaction of these residues. Moreover, the results suggest that one subunit in pH-induced closed conformation is enough to occlude the channel pore.
Taken together, these results strongly suggest a model in which Arg-41, Lys-80, and Arg-311 form a triad within one subunit that repels hydrogen ions away from the amino group of Lys-80, resulting in anomalous titration.
Shifted pH Gating in Patients with aBS Results from Structural Disturbance of the Arg-Lys-Arg Triad.
The inheritance mode of aBS is autosomal recessive, i.e., mutations in both alleles are required for the presentation of symptoms (26). Patients with aBS caused by mutations in the ROMK gene may be homozygous for the mutation, but often carry different mutations on each of the chromosomes (22, 24).
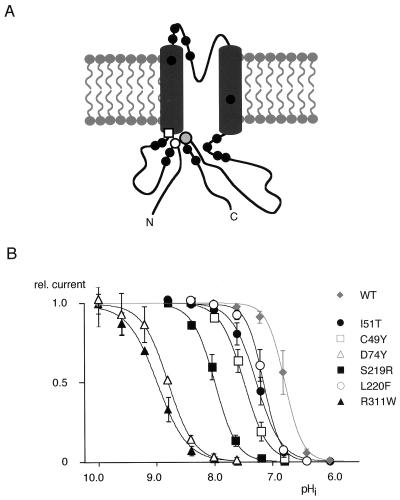
Because alterations in pH gating might well explain aberrant ROMK function under physiological conditions, we investigated the effect of aBS mutations located in the intracellular N and C termini on pH gating. Interestingly, most of these mutations are clustered around the Arg-Lys-Arg triad or in domains close to it when considered in the context of the tertiary topology deduced here for Kir proteins (Fig. 5A). As a consequence, these mutations were expected to disturb the structural arrangement of the triad, and in turn shift the pH gating off the neutral range. This shift in gating was indeed observed on heterologous expression of individual aBS mutants. As shown in Fig. 5B, most of the mutant channels exhibited current-pHi relations that were shifted to the alkaline pH range. The shift ranged from 0.4 pH units to more than 2 pH units, with the largest shifts observed for mutations of the triad residues. The ability of the channels to open and close was not affected in either of the aBS mutations investigated, nor was the cooperativity of the gating process altered significantly (Fig. 5B).
Figure 5.
Disruption of the Arg-Lys-Arg triad as a result from mutations associated with aBS shifts pH gating off the neutral pH range. (A) Tertiary topology of the Kir1.1 subunit as deduced here together with point mutations identified in patients with aBS to date. Filled circles represent aBS muations, open symbols represent residues of the Arg-Lys-Arg triad. Mutations (referred to the ROMK1 sequence) are C49Y, I51T, V72E, D74Y, W99C, D108H, P110L, V122E, N124K, G167E, L214V, S219R, L220F, R311Q/W, V315G, and F325C. (B) Current-pHi relation obtained from Kir1.1 wild type and the aBS mutations indicated. Lines represent fit of a Boltzmann function to the data (mean ± SD of 5–8 experiments); values for pH0.5 were as follows: C49Y (7.5), I51T (7.3), D74Y (9.0), S219R (7.9), L220F (7.2), R311W (8.9), and V315G (6.9).
It should be emphasized that a shift in pH gating as small as 0.4 pH units is able to induce severe clinical symptoms. Thus, one patient (see Materials and Methods) combines mutations L220F (ΔpH0.5: 0.4) with R311W (ΔpH0.5: 2.1) and another one harbors mutations I51T (ΔpH0.5: 0.5) and C49Y (ΔpH0.5: 0.7). In either case, none of the parents suffered from aBS.
Taken together, these results show that most of the aBS mutations in N and C termini of the Kir1.1 protein lead to aberrant pH gating via disturbance of the Arg-Lys-Arg triad and thus present a molecular explanation for defective ROMK function in a number of patients with aBS.
Discussion
Shift in pKa as a Result from Electrostatic Interactions in the Arg-Lys-Arg Triad.
In general, pKa shifts of titratable groups relative to the free amino acid are thought to reflect the particular chemical environments within a protein, such as hydrophobic pockets, specific hydrogen bonds, or neighboring charges. For example, a shift in pKa of almost 4 pH units was assumed for either one or both of two lysine residues interacting via hydrogen bonds in ovotransferrin (33) and a shift by about 3 pH units was reported for a histidine residue embedded in a highly positively charged environment within the FK506 binding protein (34).
In Kir proteins, the side chain of the amino acid at the preM1 site appears readily accessible from the cytoplasm, because Lys-80 in Kir1.1 and Cys-84 in Kir2.1 could be chemically modified by FmocCl (Fig. 1) and methanethiosulfonate reagents (MTSEA and MTSES, respectively; ref. 18). Therefore, hydrophobic shielding of the gating-triggering lysine is rather unlikely. Instead, the lysine residue seems to be embedded in a positively charged environment formed by Arg-41 and Arg-311. These arginines are likely permanently charged, because titration of the guanidino group occurs at more alkaline pH values (nominal pKa of 12.5; ref. 35) than that of the amino group, and Arg-311 could be replaced by lysine but not by an uncharged glutamine (Fig. 3). The Arg-Lys mutations at positions 41 and 311 further show that not only the charge of these side chains but also their location relative to the amino group of the lysine is critical for pH gating (Fig. 5). Based on these results, we concluded that Lys-80, Arg-41, and Arg-311 are located in close spatial proximity, where the positively charged arginines establish a local field repelling hydrogen ions from the amino group of the lysine residue. This field does not interfere with the transmembrane electrical field, because pH gating was the same whether recorded at −80 mV or 50 mV (not shown). Further support for electrostatic interactions as the mechanism underlying the pKa shift comes from recent experiments by Choe et al. (21) who found that introduction of a lysine or glutamate at position 51 in ROMK2 (equivalent to residue 70 in ROMK1) shifted pH gating of Kir1.1 channels by about 0.5 pH units to more acidic or alkaline pHi, respectively.
Mechanism of pH Gating.
pH gating of Kir channels is driven by titration of the lysine residue at the preM1 site (Fig. 1), i.e., protonation of the ɛNH2 group is a prerequisite for channel inactivation. Protonation and inactivation gating are separate processes as indicated by the R311W and R294Q mutations in Kir1.1 and Kir4.1, respectively. In both mutant channels, the gating machinery is intact, the working range of the pH sensor, however, is shifted toward alkaline pHi by more than 2 pH units (Figs. 3 and 5). The Arg-Lys-Arg triad presented here explains the molecular mechanism underlying the driving force of pH gating; the residue(s) comprising the channel gate are presently unknown.
Recently, gating induced by changes in extracellular pH (pKa around 2.5) has been related to conformational changes in the two-segment type potassium channel from Streptomyces lividans (KcsA, ref. 36). Thus, Perozo and coworkers (36) uncovered structural rearrangements at the C-terminal end of the second transmembrane helix that were hypothesized to change the width of the inner vestibule and thereby to control ion permeation. Whether such a mechanism also underlies pH gating in Kir1.1 remains to be elucidated. Alternatively, the gate may be formed by protein domains neighboring the Arg-Lys-Arg triad, because these domains including cysteines 49 and 308 were shown to move during pH gating (20).
Different from other gating processes in Kir channels such as G protein-mediated activation of GIRK channels or inhibition and activation of KATP channels by sulfonylureas and channel openers, pH gating is intrinsic to the Kir1.1 and Kir4.1 proteins, not requiring any accessory β-subunit or cofactors such as anionic phospholipids. The latter were reported to be crucial for maintenance of channel activity in excised patches (37) and to control ATP-mediated closure of KATP channels (38, 39). In our experiments Kir1.1 channels did not display channel run-down (Mg2+-free solutions), nor did application of PIP2 change steady-state parameters of pH gating (values for pH0.5 and Hill coefficient were 6.8 and 2.9 and 6.7 and 2.4 in the absence and presence of PIP2, respectively). Furthermore, pH gating also was observed in Kir1.1(R188Q) mutant channels, which were found to have reduced binding affinity for PIP2 (37). The mild shift in pH0.5 found for this mutant (pH0.5 of 7.4) was similar to that seen in other N- and C-terminal mutations (Fig. 2), including mutations that removed the serine residues (S44A, S219A/R, S313A) previously identified as target sites for protein kinase A (40). The latter suggests that pH gating may be modulated by protein phosphorylation.
Implication of the Arg-Lys-Arg Triad for the Tertiary Folding of Kir Proteins.
pH gating was successfully introduced into members of all known Kir subfamilies by mutating the preM1 site to a lysine residue (ref. 18; unpublished observations). Although the respective pH0.5 values displayed some variability [pH0.5 values were 6.8 for Kir1.1, 7.1 for Kir2.1(M84K), 6.1 for Kir4.1, and 7.4 for Kir6.2(T71K)], titration of the amino group in either subtype was shifted by more than 3 pH units compared with free lysine. Thus, all Kir proteins contain the structural context necessary for formation of the Arg-Lys-Arg triad and the associated gating machinery. Indeed both Arg residues are conserved throughout the Kir family and reside within a region where Kir proteins show a very high degree of homology (6). Thus, our results suggest a tertiary folding of Kir proteins such that the N and distal C termini are backfolded to the preM1 region (Fig. 5A).
Significance of pH Gating for aBS.
Point mutations identified in the ROMK gene of aBS patients are scattered throughout the amino acid sequence of Kir1.1 (24, 26). However, when considered in the context of the model for tertiary folding presented here, most aBS mutations fall into two categories: (i) mutations in the inner core region and (ii) mutations in or close to the determinants of the Arg-Lys-Arg triad (Fig. 5A). In heterologous expression experiments, category i mutants did not lead to channel activity (27), whereas most of the category ii mutants tested encoded functional channels. Their pH gating, however, was shifted to more alkaline pH values compared to wild type, with shifts in pH0.5 ranging from about 0.4 pH units (e.g., L220F) to >2 pH units (e.g., R311W, R311Q) (see Figs. 2, 3, and 5). These shifts in pH gating may result either from disruption of the Arg-Lys-Arg triad (R311Q/W) or from mutation-induced structural disturbance of this arrangement.
Taken together, altered pH gating suggests a new molecular mechanism for the pathophysiology of aBS and may open up a new perspective for the treatment of at least some of the patients.
Acknowledgments
We thank Dr. H. Echner for advice in modification of amino acids and Drs. C. Rudin and C. Mache for providing DNA of some aBS patients. We are indebted to Drs. T. Baukrowitz, J. Mosbacher, D. Oliver, and S. Waldegger for stimulating discussions and reading the manuscript. The work was supported by the Deutsche Forschungsgemeinschaft (Ru 535/4-2).
Abbreviations
- SPM
spermine
- aBS
antenatal Bartter syndrome
- FmocCl
9-fluorenylmethoxycarbonyl-chloride
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wang W, Sackin H, Giebisch G. Annu Rev Physiol. 1992;54:81–96. doi: 10.1146/annurev.ph.54.030192.000501. [DOI] [PubMed] [Google Scholar]
- 2.Giebisch G. Am J Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 3.Hebert S. Am J Physiol. 1998;275:F325–F327. doi: 10.1152/ajprenal.1998.275.3.F325. [DOI] [PubMed] [Google Scholar]
- 4.Ho K, Nichols C G, Lederer W J, Lytton J, Vassilev P M, Kanazirska M V, Hebert S C. Nature (London) 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 5.Nichols C, Lopatin A. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 6.Doupnik C A, Davidson N, Lester H A. Curr Opin Neurobiol. 1995;5:268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 7.Glowatzki E, Fakler G, Brändle U, Rexhausen U, Zenner H-P, Ruppersberg J P, Fakler B. Proc R Soc London Ser B. 1995;261:251–261. doi: 10.1098/rspb.1995.0145. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Jan Y N, Jan L Y. Neuron. 1995;15:1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 9.Raab-Graham K, Vandenberg C. J Biol Chem. 1998;273:19699–19707. doi: 10.1074/jbc.273.31.19699. [DOI] [PubMed] [Google Scholar]
- 10.Krapivinsky G, Gordon E A, Wickman K, Velimirovic B, Krapivinsky L, Clapham D E. Nature (London) 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 11.Fakler B, Brändle U, Glowatzki E, Weidemann S, Zenner H-P, Ruppersberg J P. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- 12.Ficker E, Taglialatela M, Wible B A, Henley C M, Brown A M. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- 13.Lopatin A N, Makhina E H, Nichols C G. Nature (London) 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 14.Boim M A, Ho K, Shuck M E, Bienkowski M J, Block J H, Slightom J L, Yang Y, Brenner B M, Hebert S C. Am J Physiol. 1995;268:F1132–F1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Tate S S, Palmer L G. Am J Physiol. 1994;263:C809–C824. doi: 10.1152/ajpcell.1994.266.3.C809. [DOI] [PubMed] [Google Scholar]
- 16.Lee W-S, Hebert S. Am J Physiol. 1995;268:F1124–F1131. doi: 10.1152/ajprenal.1995.268.6.F1124. [DOI] [PubMed] [Google Scholar]
- 17.Doi T, Fakler B, Schultz J H, Brändle U, Weidemann S, Zenner H P, Lang F, Ruppersberg J P. J Biol Chem. 1996;271:17261–17266. doi: 10.1074/jbc.271.29.17261. [DOI] [PubMed] [Google Scholar]
- 18.Fakler B, Schultz J H, Yang J, Schulte U, Brändle U, Zenner H P, Jan L Y, Ruppersberg J P. EMBO J. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai T D, Shuck M E, Thompson D P, Bienkowski M J, Lee K S. Am J Physiol. 1995;37:C1173–C1178. doi: 10.1152/ajpcell.1995.268.5.C1173. [DOI] [PubMed] [Google Scholar]
- 20.Schulte U, Hahn H, Wiesinger H, Ruppersberg J, Fakler B. J Biol Chem. 1998;273:34575–34579. doi: 10.1074/jbc.273.51.34575. [DOI] [PubMed] [Google Scholar]
- 21.Choe H, Zhou H, Palmer L, Sackin H. Am J Physiol. 1997;273:F516–F529. doi: 10.1152/ajprenal.1997.273.4.F516. [DOI] [PubMed] [Google Scholar]
- 22.Simon D, Karet F, Hamdan J, DiPietro A, Sanjad S, Lifton R. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 23.Simon D, Karet F, Rodriguez-Soriano J, Hamdan J, DiPietro A, Trachtman H, Sanjad S, Lifton R. Nat Genet. 1996;13:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 24.Karolyi L, Konrad M, Köckerling A, Ziegler A, Zimmermann D K, Roth B, Wieg C, Grzeschik K, Koch M, Seyberth H W, et al. Hum Mol Genet. 1997;6:17–26. [Google Scholar]
- 25.Seyberth H, Soergel M, Köckerling A. Hypokalemic Tubular Disorders: The Hyperprostaglandine E Syndrome and Gitelman-Bartter Syndrome. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 26.Karolyi L, Koch M, Grzeschik K, Seyberth H. J Mol Med. 1998;76:317–325. doi: 10.1007/s001090050223. [DOI] [PubMed] [Google Scholar]
- 27.Derst C, Konrad M, Köckerling A. Biochem Biophys Res Commun. 1997;203:641–645. doi: 10.1006/bbrc.1996.6024. [DOI] [PubMed] [Google Scholar]
- 28.Derst C, Wischmeyer E, Preisig-Muller R, Spauschus A, Konrad M, Hensen P, Jeck N, Seyberth H, Daut J, Karschin A. J Biol Chem. 1998;273:23884–23891. doi: 10.1074/jbc.273.37.23884. [DOI] [PubMed] [Google Scholar]
- 29.Dent J, Polson A, Klymkowsky M. Development (Cambridge, UK) 1989;105:61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- 30.Carpino L, Han G. J Am Chem Soc. 1970;92:5748–5794. [Google Scholar]
- 31.Fakler B, Ruppersberg J P. Cell Physiol Biochem. 1996;6:195–209. [Google Scholar]
- 32.Stanfield P R, Davies N W, Shelton I A, Sutcliffe M J, Khan I A, Brammar W J, Conley E C. J Physiol (London) 1994;478:1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewan J, Mikami B, Hirose M, Sacchettini J. Biochemistry. 1993;16:11963–11968. doi: 10.1021/bi00096a004. [DOI] [PubMed] [Google Scholar]
- 34.Yu L, Fesik S. Biochim Biophys Acta. 1994;1209:24–32. doi: 10.1016/0167-4838(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 35.Dawson R, Elliot D, Elliot W, Jones K. Data for Biochemical Research. 3rd Ed. London: Oxford Univ. Press; 1986. [Google Scholar]
- 36.Perozo E, Cortes D, Cuello L. Nat Struct Biol. 1998;5:459–469. doi: 10.1038/nsb0698-459. [DOI] [PubMed] [Google Scholar]
- 37.Huang C-L, Feng S, Hilgeman D. Nature (London) 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 38.Shyng S, Nichols C. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- 39.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker S, Ruppersberg J, Fakler B. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z-C, Yang Y, Hebert S C. J Biol Chem. 1996;271:9313–9319. doi: 10.1074/jbc.271.16.9313. [DOI] [PubMed] [Google Scholar]