Abstract
Objective
The intracellular signals that contribute to G-CSF receptor induced stem cell mobilization are poorly characterized.
Methods
We show enhanced G-CSF induced mobilization of stem cells in mice deficient in the expression of Src family kinases (SFK−/−), which is associated with hypersensitivity of SFK−/− bone marrow cells to G-CSF as well as sustained activation of Stat3.
Results
A proteome map of the bone marrow fluid derived from wildtype and SFK−/− mice revealed a significant global reduction in the number of proteins in SFK−/− mice compared to controls, which was associated with elevated MMP-9 levels, reduced SDF-1 expression, and enhanced break down of VCAM-1. Transplantation of wildtype or SFK−/− stem cells into wildtype mice and treatment with G-CSF recapitulated the G-CSF induced increase in stem cell mobilization noted in SFK−/− non-transplanted mice; however, the increase was significantly less. G-CSF treatment of SFK−/− mice engrafted with wildtype stem cells also demonstrated a modest increase in stem cell mobilization compared to controls, however the observed increase was greatest in mice completely devoid of SFKs.
Conclusions
These data suggest an involvement of both hematopoietic intrinsic and microenvironmental factors in Src kinase mediated mobilization of stem cells and identify Src kinases as potential targets for modulating stem cell mobilization.
Introduction
Hematopoietic stem and progenitor cells (HSC/Ps) contribute to the maturation and self-renewal of all blood lineages. In adults, majority of HSC/Ps reside in the bone marrow. However, a significant proportion of these cells circulate continuously between peripheral blood (PB) and bone marrow (BM) throughout an adult’s life span, a process commonly referred to as “mobilization” [1,2]. HSC/P mobilization can be significantly enhanced by administering humans or mice growth factors such as granulocyte-colony stimulating factor (G-CSF). In general, injecting G-CSF in either humans or mice results in a time and a dose-dependent neutrophilia, followed by an increase in the mobilization of identifiable clonogenic HSC/Ps in peripheral blood. Although, G-CSF induced peripheral blood mobilization of HSC/Ps is the most common mechanism of collecting stem cells for purposes of transplantation; mobilization tends to be variable, and a signficant (up to 5%) proportion of individuals do not respond to G-CSF treatment or respond poorly [3,4]. Thus, identifying alternate methods of mobilizing stem cells and/or understanding the signals downstream of the G-CSF receptor may allow for the development of additional strategies to mobilize stem cells in individuals that do not respond to G-CSF or respond poorly to G-CSF adminstration.
Neutrophils play an indispensable role in HSC/P mobilization. Blocking the function of neutrophils by administrating an anti-GR-1 antibody inhibits G-CSF induced mobilization of HSC/Ps [5,6]. Consistently, deficiency of G-CSF receptor in mice also impairs HSC/P mobilization [7]. Interestingly, G-CSF adminstration of mice harboring both the wildtype (normal cells) and the G-CSF receptor deficient cells equally mobilize normal as well as G-CSFR deficient HSC/P, suggesting that G-CSF induced mobilization requires normal/functioning neutrophils, but does not depend on the expression of G-CSF receptor on HSC/Ps [7]. It is generally believed that mobilization of HSC/Ps correlates with the release of active proteases by neutrophils in response to G-CSF [8,9]. Proteases such as neutrophil elastase (NE), cathepsin G (CG) and MMP-9 are rapidly released into the bone microenvironment in response to G-CSF treatment [8,9]. Importantly, specific inhibitors of these proteases inhibit the mobilization of HSC/Ps and treating mice with serpina, an inhibitor of both NE and CG, completely blocks HSC/P mobilization [6,10,11]. Likewise, antibodies against MMP-9 also partially inhibit G-CSF induced mobilization of HSC/Ps [6,12]. Taken together, G-CSF induced release of proteases by neutrophils is a critical step in the mobilization of stem cells. However, the nature of intracellular signals downstream from the G-CSF receptor that contribute to a highly proteolytic bone marrow meleu is poorly understood.
Proteases have been shown to induce the breakdown of a number of molecules that play an essential role in the retention of stem cells in the bone marrow microenvironment, including the breakdown of adhesion molecules such as VCAM-1 and chemokines such as SDF-1 [8,9,13–15]. Consistently, loss of VCAM-1 expression in the bone marrow or transplantation of CXCR4 (the receptor for SDF-1) deficient fetal liver cells into a lethally irradiated recipient results in enhanced circulation of HSC/Ps in the peripheral blood [16–21]. Although these studies suggest that perturbation of VCAM-1 interactions in the BM is sufficient to induce mobilization, recent studies performed in mice deficient in the expression of NE and CG demonstrate no breakdown of VCAM-1, but show a significant degree of HSC/P mobilization, suggesting that SDF-1/CXCR4 interactions may play a more prominent role in the mobilization of stem cells in these mice [13]. Consistently, SDF-1 levels have been shown to decrease during G-CSF mobilization and the reduction in the levels of SDF-1 correlate with the degree of mobilization [10,22]. The reduction in SDF-1 levels in the marrow cavity also correlates with maximum proteolytic activity. Several studies have now clearly established that the reduction in SDF-1 levels during G-CSF treatment is at least in part due to enhanced proteolysis, although more recent studies have suggested an essential role for osteoblasts/osteoclasts in regulating SDF-1 levels and thus mobilization [6,10,22]. Taken together, these results point to an essential role for neutrophil released proteases in the breakdown of SDF-1 and VCAM-1 and perhaps a more prominent role for the CXCR4/SDF-1 axis in retaining HSC/Ps in the marrow. Although it is generally agreed that a proteolytic balance in the hematopoietic microenvironment is essential for the maintenace of normal trafficking and retention of stem cells, little is known about the intracellular signals via which G-CSF regulates the trafficking and retention of stem cells in the marrow cavity. Recent studies have demonstrated that Src family kinases play an important role in negatively regulating neutrophil responses to G-CSF [23]. In the present study, we have examined the role of Src family kinases in regulating the mobilization of stem cells in response to G-CSF.
Materials and Methods
Mice
Mice lacking various members of Src family kinases (Hck, Fgr and Lyn) have been backcrossed into C57BL/6 strain (CD45.2 allele) for more than 20 generations. Congenic C57BL/6 (CD45.2 allele) and B6.BoyJ mice (CD45.1 allele) were purchased from Jackson Laboratories (Bar Harbor, ME), maintained in our animal facility, and used between 8 to 12 weeks of age. The Indiana University School of Medicine Institutional Animal Care and Use Committee approved all studies.
Purification of lineage negative (Lin−) bone marrow cells and mobilization assays
Lineage depletion was performed using the Easy Sep procedure from Stem Cell Technologies (Vancouver, BC, Canada). The purity of Lin-Sca-1+c-Kit+ cells was greater than 99%. Mobilization experiments were performed by injecting mice for 3 to 5 days with 300 μg/kg/day of human G-CSF (Amgen, CA) given at 12 hour intervals. Peripheral blood (PB), spleen, and bone marrow (BM) was obtained after the last injection and assayed for colony forming unit (CFU)-C content.
Peripheral blood, spleen, and bone marrow progenitor growth
PB progenitors were prepared using 100 μls of PB per ml. Red cells were lysed with red cell lysis buffer (160 mM NH4Cl, 100 mM KHCO3, and 0.1 mM EDTA) for 5 minutes at 4°C. Lysis was stopped using complete medium (IMDM, 10% FBS, 1% P/S, and 2 mM L-glutamine). Cells were washed twice and plated in methylcellulose (Methocult M3434) supplemented with cytokines. Cultures were incubated at 37°C in 5% CO2 for 7 to 10 days, and colonies were classified as burst forming unit-erythroid (BFU-E), colony forming unit-granulocyte macrophage (CFU-GM), or CFU-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM) and were totaled and reported as CFU-C.
Preparation of bone marrow fluid from SFK−/− mice
Tibias, iliac crest and femur bones of 6 to 8 week old SFK−/− mice or wildtype mice were isolated. All bones were flushed with two-dimensional electrophoresis compatible buffer consisting of 0.05 M Tris pH 7.2 and centrifuged to remove cells at 2000 rpm for 1 minute at 4°C. The bone marrow fluid samples were processed immediately or stored at −80°C. Two-dimensional gel electrophoresis (2-DE) was performed according to manufacturer’s instructions (Bio-Rad, Hercules, CA).
Differential expression profiling of the bone marrow fluid in SFK−/− mice
The coomassie blue stained two-dimensional gels were scanned and digitized under visible light using the Flour-S MAX Multi Imager system (Bio-Rad). Images were analyzed using PDQuest image analysis software (Bio-Rad). The image analysis software was used to create a Match Set consisting of all the gels to be analyzed followed by generating a reference template that contains the spot data from all the gels in the Match Set. Each gel in the Match Set was matched to the reference template, with landmark proteins (those uniformly expressed in all of the gels) used to quickly and accurately match all gels. The abundance of individual proteins was calculated using a quantitative analysis set within the PDQuest software, which allows for the construction of a fold change comparison chart for any given expressed protein.
Analysis of degradation of SDF-1 and VCAM-1 by bone marrow extracellular fluid and inhibitor studies
BM extracellular fluid was obtained by flushing femurs of G-CSF treated or phosphate buffered saline (PBS) treated mice with 500 μls of ice-cold PBS and then collecting the cell free supernatant following centrifugation at 500g for 2 minutes. In vitro breakdown of SDF-1 and VCAM-1 was performed as previously described [13]. Amount of MMP-9 present in the extracellular fluid of mice treated with PBS or G-CSF was measured by gelatin zymography as previously described [13]. For in vitro inhibitor studies, bone marrow fluid was harvested as described above. 20 μls of BM extracellular fluid was pre-incubated for 15 minutes at 37°C with 1 mg/ml of human α1-antitrypsin (Sigma-Aldrich, St Louis, Missouri), 1 mM PMSF (Sigma), 1 mM O-phenantroline (Sigma) and 1 μM MMP-9/MMP-13 inhibitor. 5 ng of recombinant VCAM-1 was added for digestion at 37°C for 1 hour. VCAM-1 breakdown was analyzed by western blot analysis.
Bone marrow transplantation and chimerism analysis
10 to 12 week old mice were lethally irradiated (1100 cGy split dose) before transplantation. Low density bone marrow (LDBM) [1 × 106] from wildtype or SFK−/− mice were resuspended in 0.2 ml of IMDM 2% FCS and injected into the tail vein of lethally irradiated recipients. Tail vein blood samples (100 μls) were obtained monthly post-transplantation for analysis of chimerism and multilineage analysis. Each sample was stained with CD45.2-FITC (C57BL/6) and CD45.1-PE (B6.BoyJ strain) plus four individual lineage markers conjugated to PE at 4°C for 30 minutes. Samples were washed twice and resuspended in PBS 0.1% BSA before analysis by fluorescence cytometry. All data were analyzed using CELL Quest software (Beckton Dickinson).
Results
Loss of Src family kinases (SFKs) results in enhanced mobilization of HSC/Ps
To investigate the role of Src family kinases in G-CSF induced mobilization of stem/progenitor cells, we injected wildtype mice and mice deficient in the expression of three hematopoietic specific Src family kinases Hck, Fgr and Lyn (SFK−/−) with 300 μg/Kg/day of G-CSF for 4 days or PBS as control. Mice were examined for peripheral blood white cell counts and progenitor content in peripheral blood and in the spleen. A significant increase in the total number of white blood cells was observed in SFK−/− mice treated with G-CSF compared to controls (Figure 1A). Further, G-CSF treatment of SFK−/− mice resulted in a 5-fold increase in the mobilization of colony forming cells in PB compared to wildtype controls (Figure 1B). A modest, but a significant increase in the colony forming ability of SFK−/− PB was also observed under baseline conditions. Importantly, phenotypic analysis of G-CSF mobilized peripheral blood cells in SFK−/− mice revealed a signficant increase in the frequency of phenotypically defined primitive stem/progenitor cells, including Lin-Kit+Sca-1+ cells compared to wildtype controls (Figure 1D). This increase was also noted under baseline conditions (Figure 1D). In addition to G-CSF induced enhanced mobilization of stem/progenitors in the PB of SFK−/− mice, spleens of these animals also demonstrated a significant increase in the frequency and numbers of colony forming cells at baseline as well as in response to G-CSF in comparison to wildtype controls (Figure 1C). Importantly, no significant difference in the bone marrow and spleen cellularity was observed between wildtype and SFK−/− mice (data not shown). Thus, loss of SFKs results in enhanced mobilization of HSC/Ps under baseline condition and in response to G-CSF treatment.
Figure 1. G-CSF induced mobilization of hematopoietic stem/progenitor cells is increased in SFK−/− mice compared to WT mice.
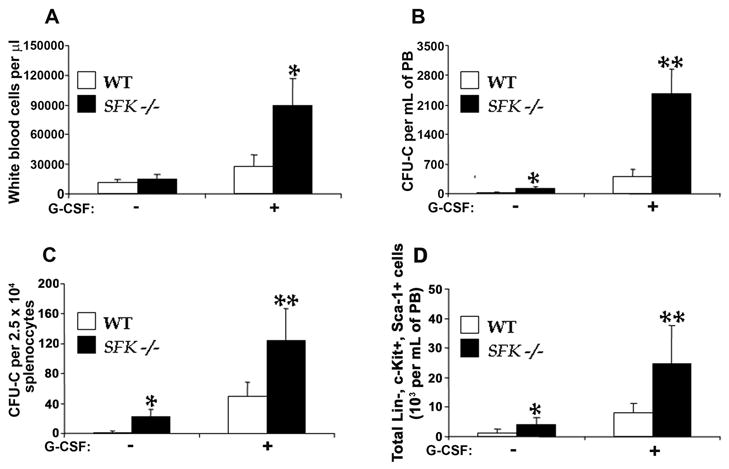
Wildtype and SFK−/− mice were injected with 300 μg/kg/day of G-CSF for 4 days at 12 hour interval. (A) 12 hours after the last injection, 100 μl of peripheral blood was harvested by tail vein bleeding and analyzed using hemavet950 instrument. Bars represent the mean of 6 independent experiments with 2–3 mice per experiment *P < 0.001, SFK−/− vs WT. (B) Wildtype and SFK−/− mice were treated with G-CSF as above, peripheral blood was harvested, and cells were lysed and plated in a methylcellulose assay in replicates of three. Data shown is the mean of 9 independent experiments with 2–4 mice per experiment **, *P < 0.001, SFK−/− vs WT. (C) Mononuclear cells (2.5 × 104) from spleens of G-CSF treated wildtype or SFK−/− mice were plated in a methylcellulose assay in replicates of three and CFU-Cs were scored 7 days later. Data shown is the mean of 8 independent experiments with 2–4 mice in each experiment **, *P < 0.001, SFK−/− vs WT. (D) Wildtype and SFK−/− mice were treated with G-CSF as above, peripheral blood was harvested, and cells were depleted of red cells and stained with antibodies against lineage markers, including Gr-1, Mac-1, B220 and CD3. Total numbers of c-Kit+Sca-1+Lin- (KSL) cells were calculated by multiplying the over all percentage of KSL cells with the peripheral white cell count. Bars denote the mean of two independent experiments **,*P<0.05, SFK−/− vs WT.
The enhanced G-CSF dependent response in SFK−/− mice prompted us to examine the ability of SFK−/− HSC/Ps to respond to G-CSF in vitro. As seen in Figure 2A, low-density bone marrow progenitors derived from SFK−/− mice showed hypersensitivity to increasing doses of G-CSF in a thymidine incorporation assay compared to wildtype controls. The hypersensitivity to G-CSF was also seen in a colony-forming assay over a 7-day culture period (Figure 2B). To determine whether G-CSF induced, hypersensitivity seen in SFK−/− HSC/Ps was intrinsic to HSC/Ps, equal number of lineage depleted HSC/Ps derived from wildtype or SFK−/− bone marrow were treated with increasing doses of G-CSF and subjected to a colony forming assay. As seen in Figure 2C, a significant increase in the number of CFU-Cs was observed in cultures derived from SFK−/− BM compared to wildtype controls. Taken together, these results support the notion that deficiency of Src family kinase in bone marrow progenitor’s results in hypersensitivity to G-CSF, which is associated with a significant increase in the mobilization of phenotypically defined primitive stem/progenitor cells.
Figure 2. Deficiency of SFKs in hematopoietic stem and progenitor cells results in hypersensitivity to G-CSF.
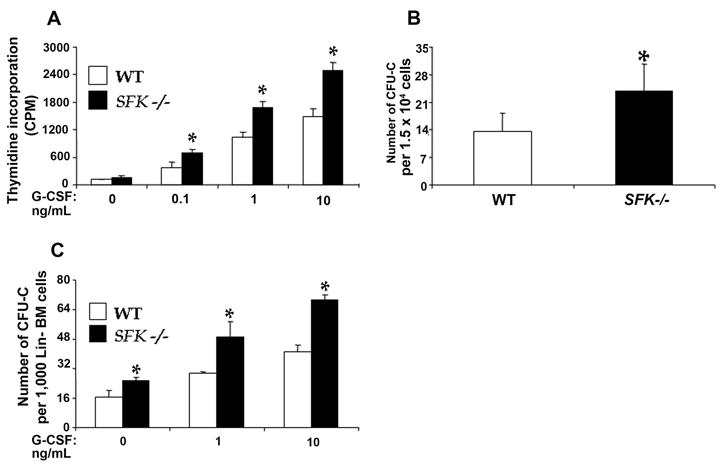
(A) Low density mononuclear cells (MNCs) were cultured in the presence of increasing concentration of G-CSF. After 48 hours and six hours prior to harvesting, cells were pulsed with [3H] thymidine. Proliferation was measured by thymidine incorporation (counts per minute). Bars denote the mean of four independent experiments. *P<0.05, WT vs SFK−/−. (B) Low density MNCs were harvested and plated in a methylcellulose assay with 10 ng of G-CSF. Colonies were scored 7 days later. Bar graph shows the mean of four independent experiments done in triplicates. *P<0.05, SFKs −/− vs WT. (C) Lineage negative cells were plated in a methylcellulose assay with varying concentrations of G-CSF. Colonies were scored 7 days later. Bars represent the mean colony forming ability from an independent experiment. *P<0.05, SFKs −/− vs WT.
Hypersensitivity to G-CSF due to SFK deficiency results in enhanced Stat3 activation
Previous studies have argued for a critical role for Stat3 in regulating G-CSF responses in HSC/Ps, including G-CSF induced mobilization [24]. To determine whether the increase in G-CSF induced mobilization of stem/progenitors in SFK−/− mice is associated with increased activation of Stat3, we isolated lineage negative cells from wildtype and SFK−/− mice and stimulated a fixed number of these cells with G-CSF for various time points up to 30 minutes. As seen in Figure 3A, stimulation of SFK−/− bone marrow cells with 10 ng/ml G-CSF resulted in a significant increase and sustained activation of Stat3 compared to wildtype controls at every time point examined. Additionally, a modest increase in the activation of Stat3 in SFK−/− HSC/Ps was also observed under basal conditions. Although, G-CSF is known to activate the Ras-MAPK as well as the PI-3Kinase/Akt pathway, activation of the Ras-MAPK or the Akt pathways was not substantially affected in these cells (data not shown). Thus, SFKs appear to specifically negatively regulate the activation of Stat3, which is associated with enhanced G-CSF induced mobilization of HSC/Ps.
Figure 3. Deficiency of SFKs in hematopoietic stem and progenitor cells results in enhanced activation of Stat3, but normal differentiation.
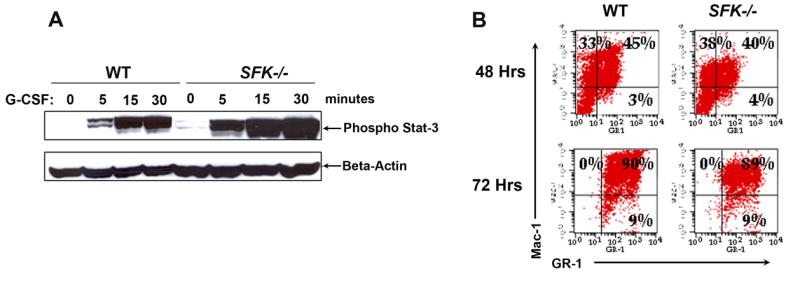
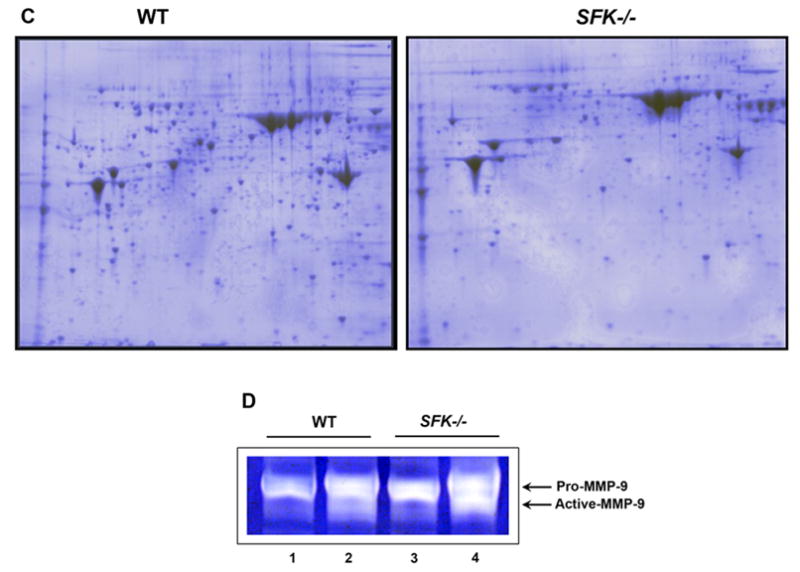
(A) Lineage negative cells from wildtype and SFK−/− mice were stimulated with G-CSF for the indicated time points. Equal amount of protein was used to run a SDS-PAGE gel and probed with an anti-phospho-Stat3 antibody. Top panel shows the amount of phospho-Stat3 protein in each lane and the bottom panel shows equal protein in each lane as assessed by the presence of β-actin. (B) Wildtype and SFK−/− Lin− bone marrow cells were in vitro differentiated. After 48 and 72 hours, cells were harvested and stained with antibodies that recognize the cell surface marker Mac-1 and Gr-1. (C) Wildtype and SFK−/− mice were injected with 300 μg/kg/day of G-CSF for 4 days and bone marrow fluid was harvested. Equal amount of bone marrow fluid (200 μg) was used to run a 2-D SDS-PAGE gels. Shown is a representative 2-D gel that was fixed, stained with coomassie blue and destained with deionized water. Similar results were seen in three independent experiments. (D) Wildtype and SFK−/− mice were injected with 300 μg/kg/day of G-CSF for 4 days and plasma was harvested. Equal amount of plasma from wildtype and SFK−/− mice was used to measure MMP-9 levels by gelatin zymography as previously described. The top arrow indicates the level of Pro-MMP-9 and the bottom arrow indicates the amount of active MMP-9. Lanes 1 & 3 contain plasma from control wildtype and SFK−/− mice. Lanes 2 & 4 contain plasma from G-CSF treated wildtype and SFK−/− mice. Similar results were observed in three independent experiments.
Since G-SCF has also been shown to regulate the differentiation of neutrophils from more primitive cells, we purified Lin− cells from the bone marrow of wildtype and SFK−/− mice and subjected them to in vitro differentiation by monitoring the acquisition of Gr-1 and Mac-1 with time. At the time of initiating the cultures (day 0), the expression of Gr-1 and Mac-1 was nearly undetectable in wildtype and SFK−/− Lin− cultures (data not shown). Differentiation, as assessed by the acquisition of Gr-1 and Mac-1 was monitored at the end of 48 and 72 hours by flow cytometry. A seen in Figure 3B, loss of SFK in Lin− cells did not alter the differentiation of SFK deficient HSC/Ps relative to wildtype controls. Thus, the enhanced activation of Stat3 in response to G-CSF in SFK−/− HSC/Ps is mainly associated with increased growth and mobilization, but not differentiation of SFK−/− HSC/Ps.
Enhanced G-CSF induced mobilization of SFK−/− HSC/Ps is associated with increased breakdown of bone marrow proteins and elevated levels of MMP-9
Mechanisms of G-CSF induced mobilization of HSC/Ps are poorly understood and are likely to be very complicated. Thus far, studies suggest the involvement of both protease dependent and independent mechanism(s) of stem cell mobilization by G-CSF. In the present study, we sought to determine the impact of G-CSF on the global breakdown of proteins in the bone marrow of SFK−/− and wildtype mice. We used a 2-DE gel approach to profile the protein content in the BM of SFK−/− and wildtype mice treated with G-CSF for four days with 300 μg/Kg/day. We hypothesized that the enhanced G-CSF induced mobilization of HSC/Ps in SFK−/− mice might be associated increased breakdown of proteins in the BM fluid of these animals compared to wildtype controls. As seen in figure 3C, the number of coomassie blue spots in wildtype mice treated with G-CSF was significantly greater than SFK−/− mice treated for the same duration and dose. Although indirect, these results are suggestive of a highly proteolytic environment in the BM of G-CSF treated SFK−/− mice compared to G-CSF treated wildtype control animals. To more directly test this notion, we examined the level of a specific proteolytic enzyme MMP-9 in G-CSF treated wildtype and SFK−/− mice. As seen in Figure 3D, the reduced numbers of protein spots in the bone marrow fluid of SFK−/− mice treated with G-CSF was associated with elevated activity of proteolytic enzyme MMP-9 in these animals. A modest increase in the level of MMP-9 was also observed in SFK−/− mice under basal conditions. Taken together, these results provide both an indirect (global reduction in the number of proteins in the BM fluid) as well as direct (increase levels of MMP-9) evidence that hypersensitivity to G-CSF in HSC/Ps deficient in the expression of SFKs induces a bone marrow microenvironment that is significantly more than wildtype controls.
With respect to the major players implicated in regulating proteolytic enzyme dependent G-CSF mobilization of HSC/Ps, studies thus far have implicated a major role for VCAM-1 and SDF-1. We next sought to assess the extent to which the highly proteolytic microenvironment in SFK−/− BM contributes to the breakdown of VCAM-1 and SDF-1. We first performed in vitro experiments to determine if the enhanced G-CSF mobilization in SFK−/− mice was associated with increased breakdown of VCAM-1, and if treating mice with VCAM-1 inhibitor alters the mobilization characteristics of SFK−/− mice. Bone marrow fluid from wildtype and SFK−/− G-CSF treated mice was harvested and incubated with recombinant VCAM-1 in the presence of various inhibitors. As seen in Figure 4A, incubating equal concentration of recombinant VCAM-1 with a fixed amount of G-CSF treated BM fluid resulted in signficantly more breakdown of VCAM-1 in the presence of BM fluid derived from SFK−/− mice compared to wildtype controls. Importantly, incubating recombinant VCAM-1 in the presence of α-anti-tyrpsin significantly inhibited the breakdown of VCAM-1 in wildtype and SFK−/− BM fluids. A similar rescue in the breakdown of VCAM-1 was also noted in cultures incubated with PMSF (Figure 4A; lane 8). No other inhibitors, including the MMP9/MMP13 or o-phenanthroline rescued the breakdown of VCAM-1 (Figure 4A; lanes 9 &10). Importantly, and consistent with the basal increase in the mobilization of HSC/Ps in SFK−/− mice compared to wildtype controls, VCAM-1 breakdown in the BM fluid derived from SFK−/− mice at basal conditions was also elevated compared to wildtype controls (Figure 4A; lane 1). To determine whether treating mice in vivo with an inhibitor that completely inhibits the breakdown of VCAM-1 in vitro, would inhibit G-CSF induced mobilization of SFK−/− HSC/Ps, we treated wildtype and SFK−/− mice with α-anti-trypsin and enumerated the progenitor content in the PB of these mice. As seen in Figure 4B, treatment of SFK−/− mice with α-anti-trypsin did not inhibit G-CSF induced mobilization (Figure 4B), although degradation of VCAM-1 in vitro was clearly rescued (Figure 4A). Taken together, these results suggest that although the breakdown of VCAM-1 in G-CSF treated SFK−/− mice is signficantly increased compared to wildtype mice, the increased mobilization in these mice is not inhibitable by α-anti-trypsin treatment.
Figure 4. Enhanced VCAM-1 breakdown in G-CSF treated SFK−/− mice is inhibited by serine protease inhibitor in vitro, however G-CSF induced mobilization in vivo is not.
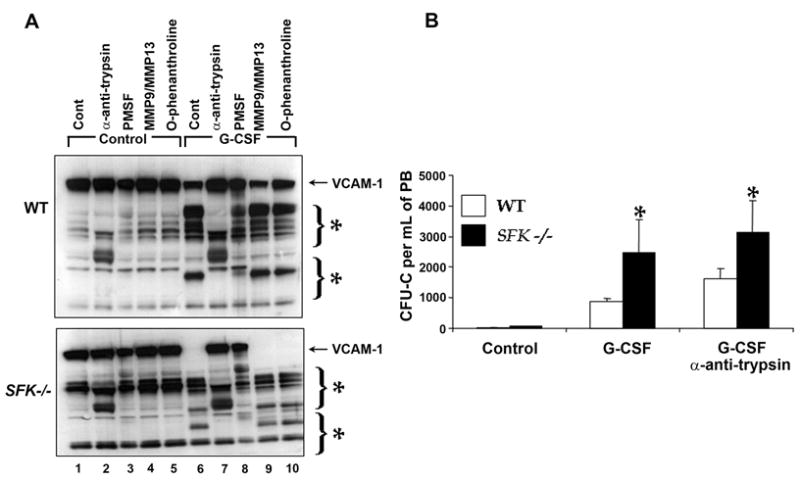
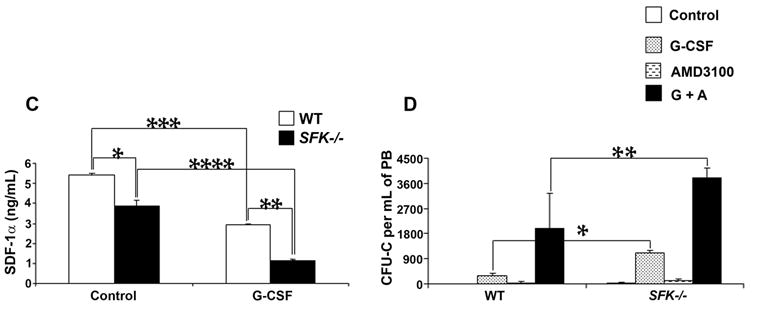
Wildtype and SFK−/− mice were injected with 300 μg/kg/day of human G-CSF for 3 days and bone marrow fluid was harvested using 500 μl of ice cold PBS. (A) Recombinant VCAM-1 (10 ngs) was incubated with bone marrow fluid for 1 hour at 37°C. For inhibitor studies, bone marrow fluid was incubated with the indicated protease inhibitors for 30 minutes before adding recombinant VCAM-1 to the reaction. Proteolytic degradation was analyzed by western blot analysis using an anti-VCAM-1 antibody. (B) 1 mg/kg/day of α-1 anti-trypsin was injected for 3 days along with 300 μg/kg/day of G-CSF. On the fourth day, peripheral blood from WT and SFK−/− mice was harvested, lysed, and plated in a methylcellulose assay in replicates of three. CFU-Cs were scored 7 days later in triplicates. Data shown is the mean from an independent experiment with 4 mice in each group. *P < 0.001, SFK−/− vs WT. (C) Wildtype and SFK−/− mice were injected with 300 μg/kg/day of human G-CSF for 4 days and their bone marrow fluid harvested. SDF-1 concentration was measured by ELISA according to manufactures recommendation. Bars indicate the mean SDF-1 level from an experiment performed in replicates of three. *P<0.05, WT vs SFK−/− (control), **P<0.05 WT vs SFK−/− (G-CSF treated), ***P<0.05 WT (control) vs WT (G-CSF) and ****P<0.05 SFK−/− (control) vs SFK−/− (G-CSF). (D) Enhanced AMD3100 induced stem/progenitor cell mobilization and cooperation with G-CSF in SFK−/− mice. SDF-1 antagonist AMD3100 was injected 1 hr before harvesting peripheral blood (PB) or 1 hr before harvesting PB from mice injected with G-CSF (300 μg/kg/day) for three days. Cells were plated in a methylcellulose assay. CFU-Cs were scored 7 days later. Bars indicate the mean colony forming ability from two independent experiments performed in replicates of three with 4 mice in each experiment. *P<0.05, G-CSF treated WT vs SFK−/− mice. **P<0.05, G-CSF+AMD3100 treated WT vs SFK−/− mice. ***P<0.05 AMD3100 treated WT vs SFK−/− mice.
Previous studies have suggested a more prominent role for the CXCR4/SDF-1 axis in the retention of HSC/Ps in the bone marrow under basal conditions as well as in response to G-CSF treatment. To determine if the enhanced G-CSF induced mobilization in SFK−/− mice is associated with reduced levels of SDF-1 in the BM of SFK−/− mice, we examined SDF-1 levels in the bone marrow of SFK−/− and wildtype mice treated with PBS or with G-CSF. As seen in Figure 4C, a significant reduction in the level of SDF-1 was observed in SFK−/− bone marrow under basal conditions as well as upon G-CSF treatment in comparison to wildtype controls.
Since disruption of the SDF-1/CXCR4 pathway is one of the principal pathways involved in the mobilization of HSC/Ps, we hypothesized that co-administering G-CSF and an SDF-1 antagonist AMD3100 would further augment the mobilization of HSC/Ps in SFK−/− mice compared to wildtype controls. Consistent with this notion, injecting SFK−/− mice with G-CSF and AMD3100 resulted in a signficant increase in the mobilization of SFK−/− HSC/Ps compared to wildtype mice treated with the same combination or mice treated with G-CSF alone (Figure 4D). These results strongly implicate the SDF-1/CXCR4 axis in negatively regulating G-CSF induced mobilization of HSC/Ps in SFK−/− mice.
Peripheral blood mobilized HSC/Ps in SFK−/− mice possess long-term and multilineage repopulating ability
Although the above studies demonstrate a signficant increase in the mobilization of clonogenic HSC/Ps in SFK−/− mice treated with G-CSF, they do not provide evidence of stem cell function. To assess whether PB mobilized HSC/Ps in SFK−/− mice possess stem cell properties, we harvested peripheral blood from G-CSF treated wildtype or SFK−/− mice and transplanted equal numbers of mononuclear cells into wildtype lethally irradiated recipients. As seen in Figure 5A, lethally irradiated recipient mice transplanted with PB derived from G-CSF treated SFK−/− mice demonstrated 100% viability 7 months post transplantation. These mice continue to survive 12 month post transplantation (data not shown). Furthermore, a comparison of the peripheral blood indices between SFK−/− non-transplanted mice and lethally irradiated recipient mice transplanted with SFK−/− PB revealed no significant differences (Figure 5B). In addition, G-CSF mobilized SFK−/− PB cells upon transplantation into a lethally irradiated wildtype recipient resulted in multilineage engraftment six months post transplantation (Figure 5C). Modest alterations in lineage distribution (B220 and Gr-1+ cells) in mice receiving SFK−/− PB cells (Figure 5C) have been previously reported in the SFK−/− non-transplanted mice [25]. These results, along with the enhanced numbers of CFU-Cs and increased frequency of KSL cells in G-CSF mobilized PB of SFK−/− mice suggests that deficiency of Src family kinases induces enhanced mobilization of stem cells that are capable of long-term and multilineage engraftment in a lethally irradiated recipient. While the present experimental design confirms the presence of stem cells in the PB of G-CSF mobilized SFK−/− mice; the current design does not provide a quantitative measure of increased numbers of stem cells in the PB of SFK−/− mice relative to wildtype.
Figure 5. G-CSF induces peripheral blood mobilization of SFK−/− stem cells capable of multi-lineage engraftment.
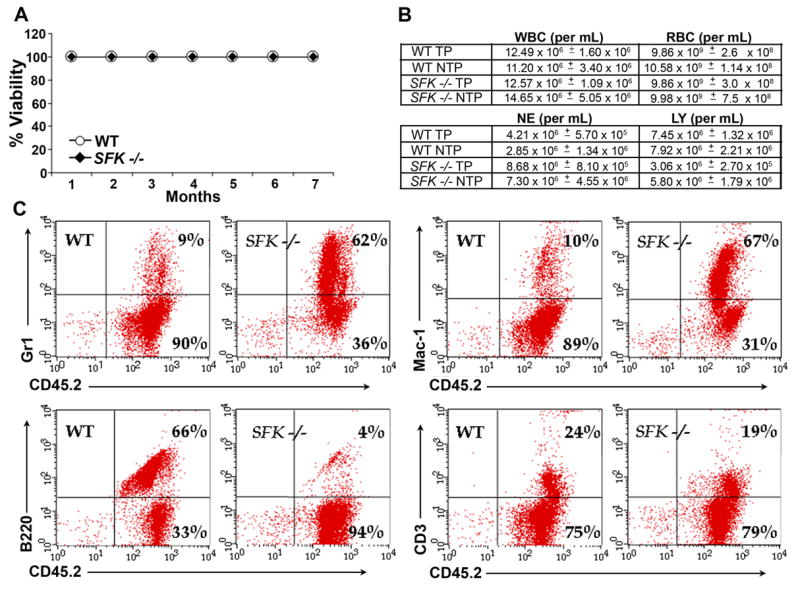
Wildtype and SFK−/− mice (20 each) were injected with 300 μg/kg/day of human G-CSF for 4 days. Peripheral blood (8 ml) was harvested and 1 ml equivalent of PB was injected into each lethally irradiated recipient. (A) Shown is a survival curve. n=7 in each group. (B) A comparison of the peripheral blood cell counts between the original non transplanted mice (NTP) versus lethally irradiated mice transplanted (TP) with G-CSF mobilized PB HSC/Ps. n=7 in each group. (C) 1 ml equivalent of G-CSF mobilized PB from wildtype or SFK−/− mice was transplanted into lethally irradiated recipients and multi-lineage engraftment in the peripheral blood of recipient mice was enumerated six months post transplantation by flow cytometry using antibodies that recognize donor cells (CD45.2) and a combination of Gr-1, Mac-1, B220 and CD3 antigens. Shown are dot blots from a representative experiment.
Enhanced G-CSF induced mobilization of stem cells in SFK−/− mice is contributed by both intrinsic and microenvironmental factors
To determine whether the increase in G-CSF induced mobilization of stem cells in SFK−/− mice is solely contributed by defects in the HSC/P (HSC/P intrinsic factors) cell compartment, we harvested low density bone marrow cells from wildtype and SFK−/− mice and transplanted them into lethally irradiated wildtype recipients. Four months post transplantation (once stable engraftment was achieved); cohorts of mice were treated with G-CSF as described above. As controls, non-transplanted wildtype and SFK−/− mice were also treated with G-CSF at the same time as transplanted mice. Figure 6A demonstrates the level of donor cell engraftment in lethally irradiated recipient mice as assessed by the expression of cell surface marker CD45.2 (donor-derived cells). As seen in Figure 6B, treating wildtype or SFK−/− transplanted or non-transplanted mice with G-CSF resulted in a signficant increase in white blood cell counts relative to wildtype controls. Consistent with previous results, the increase in white cell counts was greater in mice harboring SFK−/− HSC/Ps compared to mice harboring wildtype HSC/Ps. However, compared to G-CSF induced increase in white cell counts and mobilization of stem cells in non-transplanted SFK−/− mice, mobilization was significantly dampened in wildtype mice that were transplanted with SFK−/− HSC/Ps and treated with G-CSF (Figure 6B). These results suggest that factors intrinsic to the hematopoietic compartment contribute to G-CSF induced mobilization in SFK−/− mice; however, microenvironmental factors must also contribute to this process.
Figure 6. G-CSF induced increased mobilization of stem/progenitors seen in SFK−/− mice is also observed in lethally irradiated wildtype recipients transplanted with SFK−/− bone marrow cells.
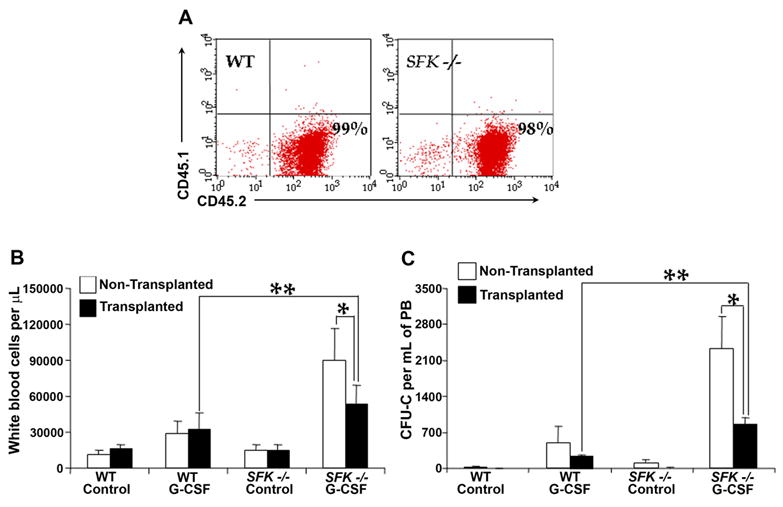
1×106 wildtype or SFK−/− bone marrow cells were injected into lethally irradiated wildtype recipients and subjected to G-CSF induced mobilization 4 months post transplantation. (A) A representative dot blot demonstrating the level of donor chimerism in recipient mice 4 months post transplantation. (B) & (C) A comparison of G-CSF induced (300 μg/kg/day for 4 days) peripheral blood cell counts and CFU-Cs in non-transplanted and transplanted mice. n=3 per group. **P<0.05, transplanted mice treated with G-CSF (WT vs SFK−/−). *P<0.05, non TP vs TP G-CSF treated SFK−/− mice.
To determine the relative contribution of the SFK−/− bone marrow microenvironment in G-CSF induced mobilization of stem cells, we performed a series of reciprocal transplant experiments using wildtype and SFK−/− bone marrow cells and lethally irradiated wildtype or SFK−/− recipient mice. Cohorts of stabley engrafted (with wildtype or SFK−/− HSC/Ps) wildtype or SFK−/− mice were treated with G-CSF as described above and enumerated for peripheral white blood cell counts and CFU-C content. As seen in Figure 7A, consistent with previous studies, G-CSF treatment of wildtype mice transplanted with wildtype bone marrow cells or SFK−/− bone marrow cells resulted in a signficant increase in peripheral blood white cell counts as well as in the total number of CFU-Cs. Interestingly, a modest but a signficant increase in the CFU-C content was also observed in SFK−/− mice transplanted with wildtype bone marrow cells compared to wildtype mice transplanted with wildtype BM cells and treated with G-CSF (Figure 7B). In all cases, G-CSF mediated increase in the number of CFU-Cs was highest in SFK−/− mice transplanted with SFK−/− HSC/Ps. Taken together, these results demonstrate that cooperation between HSC/Ps intrinsic and microenvironmental factors is necessary for maximum mobilization of stem cells in SFK−/− mice.
Figure 7. Intrinsic (stem/progenitor cell) and extrinsic (bone marrow microenvironmental) factors cooperate to enhance G-CSF induced mobilization of stem/progenitor cells in SFK−/− mice.
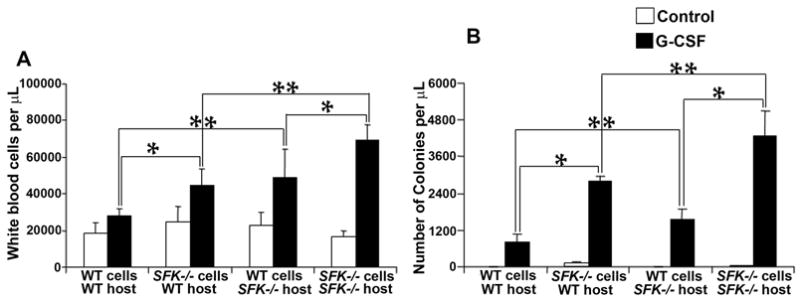
1×106 wildtype or SFK−/− bone marrow cells were transplanted into lethally irradiated wildtype or SFK−/− mice as indicated. Three to four months later, mice in different groups were injected with 300 μg/kg/day of human G-CSF for 4 days, peripheral blood was harvested and enumerated for white cell counts (A) and CFU-Cs (B). Bars represent the mean while cell counts from three independent experiments (A) or mean CFU-Cs (B) from two independent experiments with 2 to 4 mice per group in each experiment. *P<0.05, WT or SFK−/− harboring cells in WT or SFK−/− host treated with G-CSF. *P<0.05, WT cells into WT or SFK−/− host or SFK−/− cells into WT or SFK−/− host.
Discussion
In spite of flurry of recent studies documenting novel mechanism(s) of G-CSF induced mobilization of HSC/Ps, relatively little is known about the intracellular signals downstream from the G-CSF receptor that modulate some or all of the above described mechanism(s) of stem cell mobilization. A better understanding of these signals could allow us to specifically modulate certain aspects of this pathway to enhance stem cell mobilization. In this study, we have examined the role of Src family kinases in G-CSF induced mobilization of stem cells. We show enhanced G-CSF induced mobilization of stem cells in mice deficient in the expression of Src family members Hck, Fgr and Lyn (SFK−/−). This increase is associated with hypersensitivity of BM SFK−/− cells to G-CSF as well as sustained activation of Stat3. Two dimensional gel electrophoresis comparison of bone marrow fluid from G-CSF treated wildtype and SFK−/− mice revealed a significant decrease in global protein expression in SFK−/− mice compared to wildtype, suggestive of enhanced proteolysis. Detailed examination of the bone marrow fluid revealed elevated MMP-9 expression, enhanced break down of VCAM-1 and SDF-1 as well as reduced expression of SDF-1 in the bone marrow cavity of G-CSF treated SFK−/− mice compared to wildtype controls. Consistent with reduced expression of SDF-1 in SFK−/− bone marrow, injection of a SDF-1 antagonist, AMD3100 into SFK−/− mice further augmented G-CSF induced mobilization. To determine whether the increase in mobilization is intrinsic to the HSC/P compartment, we transplanted wildtype or SFK−/− HSC/Ps into lethally irradiated recipients and treated them with G-CSF. G-CSF treatment of these mice to a large extent recapitulated the initial G-CSF induced increase in mobilization seen in SFK−/− non-transplanted animals; however the increase was 50% less than that seen in the original SFK−/− mice. To determine whether the difference in mobilization between SFK−/− transplanted and non-transplanted mice is due to defective SFK−/− microenvironment, we performed a series of reverse transplant experiments, including transplanting wildtype HSC/Ps into SFK−/− mice. G-CSF treatment of these mice also showed a significant increase in mobilization compared to controls, suggesting an involvement of both HSC/P intrinsic as well as extrinsic (microenvironmental) factors in SFK mediated G-CSF mobilization. To confirm whether the G-CSF mobilized cells in SFK−/− mice were indeed stem cells, we transplanted PB cells derived from G-CSF treated SFK−/− mice into lethally irradiated animals and monitored engraftment and long-term survival. All mice demonstrated multilineage engraftment and were viable 7 months post-transplantation. While these results confirmed the presence of stem cells in the PB of G-CSF mobilized SFK−/− mice, these studies were not designed to determine if SFK−/− mice posses increased numbers of stem cells relative to wildtype.
Our results show that loss of Src family kinases in HSC/Ps results in elevated and sustained activation of Stat3 under basal conditions and in response to G-CSF, which is associated with enhanced mobilization of HSC/Ps. Interestingly, loss of SOCS3 in HSC/Ps also results in sustained and elevated activation of Stat3 in response to G-CSF, which is also associated with enhanced mobilization of HSC/Ps [24]. Our results in Src deficient HSC/Ps with respect to enhanced HSC/P mobilization are in agreement with those of Mermel et al demonstrating increased neutrophil responses in these mice in response to G-CSF [23]. Thus, Src family kinases are not only involved in emergency granulopoiesis, but also play a critical role in negatively regulating stem cell mobilization. Furthermore, our results suggest that Src family kinases, SOCS3 and Stat3 appear to function in the same biochemical pathway downstream of the G-CSF receptor.
Our results in Src kinase deficient mice demonstrating reduced levels of SDF-1 and its association with enhanced mobilization of HSC/Ps are consistent with a role for the CXCR4/SDF-1 axis in contributing to stem cell mobilization. In several independent studies, the CXCR4/SDF-1 pathway has been implicated as one of the dominant pathways in regulating the retention of stem cells in the bone marrow. Mice lacking SDF-1 or CXCR4 show impaired migration of stem cells from fetal liver to the bone marrow. Transplantation of CXCR4−/− cells results in enhanced numbers of circulating HSC/Ps in the PB. Treatment of mice with AMD3100, a CXCR4 antagonist results in rapid mobilization of stem cells. Furthermore, treating mice with G-CSF results in reduced SDF-1 levels in the bone marrow, which is associated with increased mobilization of HSC/Ps. Consistently, the greatest reduction in SDF-1 levels in the bone marrow is associated with highest mobilization of HSC/Ps. Importantly, mice in which G-CSF induced mobilization was modest, demonstrated only a modest reduction in SDF-1 levels. Taken together, these results strongly suggest a prominent role for SDF-1 in regulating G-CSF induced mobilization of stem cells and are in agreement with our data in Src deficient mice.
Although multiple mechanism(s) of G-CSF induced SDF-1 regulation have been recently proposed, including protease mediated break down of SDF-1 as well as transcriptional regulation of SDF-1 by osteoblasts, the extend to which these independent mechanism(s) of SDF-1 regulation contribute to G-CSF induced mobilization of stem cells is unclear. Our studies clearly demonstrate a significantly greater reduction in the level of SDF-1 protein in SFK−/− mice compared to wildtype control mice, which is associated with greater mobilization of stem cells compared to wildtype controls. Based on the bone marrow proteome map, it is likely that the enhanced proteolytic microenvironment of SFK−/− mice is responsible for reduced SDF-1 levels in the bone marrow of Src kinase deficient mice, although, we cannot rule out the possibility that SDF-1 levels in these mice may be regulated at the level of transcription by osteoblasts or endothelial cells. Our reverse transplant studies suggest that some aspect of the SFK−/− microenvironment also contributes to enhanced G-CSF induced stem cell mobilization, since G-CSF treatment of Src deficient mice engrafted with wildtype HSC/Ps also demonstrates a significant increase in G-CSF induced mobilization of stem cells compared to controls, although maximal mobilization of stem cells is only observed in SFK−/− mice transplanted with SFK−/− HSC/Ps. While these results suggest that some aspect of the SFK−/− microenvironment does contribute to enhanced stem cell mobilization in these mice and cooperation between HSC/P intrinsic factors, and the hematopoietic microenvironment is essential for maximal stem cell mobilization, it is unclear whether this is due to reduced expression of SDF-1 (transcriptional reduction by osteoblasts or endothelial cells) or enhanced breakdown or SDF-1 or both. Our results clearly demonstrate that Src family kinases play an essential role in regulating SDF-1 protein level in the BM microenvironment and that loss of Src family kinases reduces overall SDF-1 levels which is associated with enhanced mobilization of HSC/Ps.
Acknowledgments
Author Contribution: Jovencio Borneo – performed research & wrote paper, Veerendra Munugalavadla – performed research, Emily C. Sims – performed research, Sasidhar Vemula – performed research, Christie M. Orschell – designed research, Merv Yoder – designed research, Reuben Kapur – designed research & wrote paper
Authors also wish to thank Dr. Lowell for providing Src kinase deficient mice.
The authors of this manuscript do not have a conflict of interest or a potential for a conflict of interest.
Financial Support: R01 HL075816 (R.K.), R01 HL077177 (R.K.) & R01 HL75660 (C.O)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102:1249–1253. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 2.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 3.Villalon L, Odriozola J, Larana JG, et al. Autologous peripheral blood progenitor cell transplantation with <2 × 10(6) CD34(+)/kg: an analysis of variables concerning mobilisation and engraftment. Hematol J. 2000;1:374–381. doi: 10.1038/sj/thj/6200057. [DOI] [PubMed] [Google Scholar]
- 4.Roberts AW, DeLuca E, Begley CG, Basser R, Grigg AP, Metcalf D. Broad inter-individual variations in circulating progenitor cell numbers induced by granulocyte colony-stimulating factor therapy. Stem Cells. 1995;13:512–516. doi: 10.1002/stem.5530130508. [DOI] [PubMed] [Google Scholar]
- 5.Pruijt JF, Verzaal P, van Os R, et al. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci U S A. 2002;99:6228–6233. doi: 10.1073/pnas.092112999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103:110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000;95:3025–3031. [PubMed] [Google Scholar]
- 8.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 9.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 10.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 11.van Pel M, van Os R, Velders GA, et al. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proc Natl Acad Sci U S A. 2006;103:1469–1474. doi: 10.1073/pnas.0510192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruijt JF, Fibbe WE, Laterveer L, et al. Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9) Proc Natl Acad Sci U S A. 1999;96:10863–10868. doi: 10.1073/pnas.96.19.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levesque JP, Liu F, Simmons PJ, et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 1004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Cheng T, Olszak I, et al. CXCR-4 desensitization is associated with tissue localization of hemopoietic progenitor cells. J Immunol. 2001;166:5027–5033. doi: 10.4049/jimmunol.166.8.5027. [DOI] [PubMed] [Google Scholar]
- 15.Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 16.Foudi A, Jarrier P, Zhang Y, et al. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4−/− chimeric mice. Blood. 2006;107:2243–2251. doi: 10.1182/blood-2005-02-0581. [DOI] [PubMed] [Google Scholar]
- 17.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci U S A. 1993;90:9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779–4788. [PubMed] [Google Scholar]
- 19.Kikuta T, Shimazaki C, Ashihara E, et al. Mobilization of hematopoietic primitive and committed progenitor cells into blood in mice by anti-vascular adhesion molecule-1 antibody alone or in combination with granulocyte colony-stimulating factor. Exp Hematol. 2000;28:311–317. doi: 10.1016/s0301-472x(99)00151-4. [DOI] [PubMed] [Google Scholar]
- 20.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulyanova T, Scott LM, Priestley GV, et al. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106:86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mermel CH, McLemore ML, Liu F, et al. Src family kinases are important negative regulators of G-CSF-dependent granulopoiesis. Blood. 2006;108:2562–2568. doi: 10.1182/blood-2006-05-024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croker BA, Metcalf D, Robb L, et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 25.Saijo K, Schmedt C, Su IH, et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]


