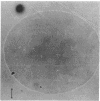
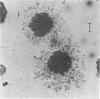
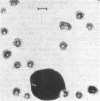
Abstract
Suspensions of enterococci were treated with lysozyme in the presence of osmotic stabilizers. The resulting osmotically fragile bodies prepared from Streptococcus faecium strain F24 and S. faecalis strain E1 gave rise to L-forms under optimal osmotic and nutritional conditions for treatment and subsequent growth. The most critical component of the growth medium, to obtain maximum yields, was the nature and concentration of the added salt. The two most effective salts were sodium chloride and ammonium chloride in the range of 2 to 3% (w/v) added to a suitable agar base. Ammonium chloride was more versatile, because it could be used with either sucrose or polyethylene glycol 4000 as the osmotic stabilizer for preparation and dilution of the osmotically fragile bodies. Sodium chloride would not consistently support growth of S. faecium F24 as L-forms when polyethylene glycol 4000 was used as the osmotic stabilizer during lysozyme treatment. Time-course studies of concurrent cell wall removal and L-form induction suggested that maximal induction required only cell wall damage rather than complete wall removal. This method for induction of L-forms from a suspension of enterococci is a significant improvement over other presently known methods.
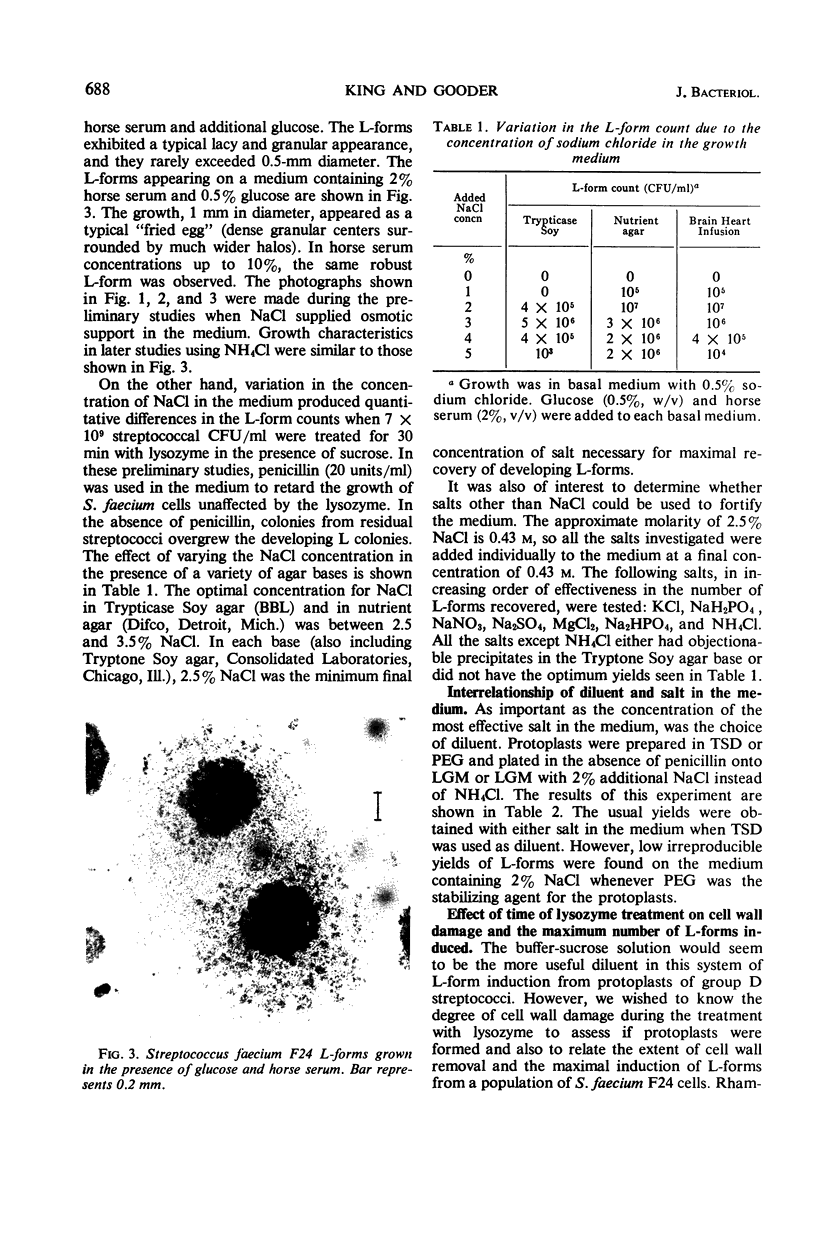
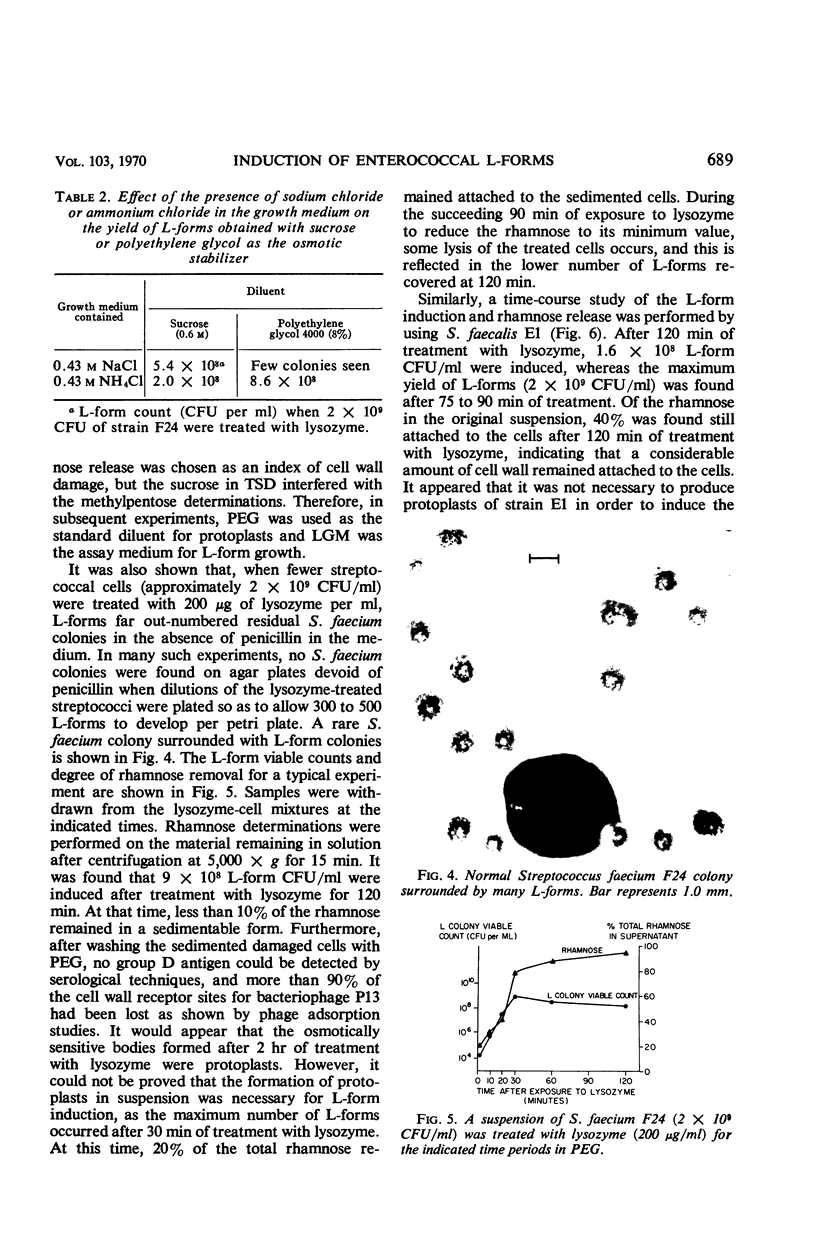
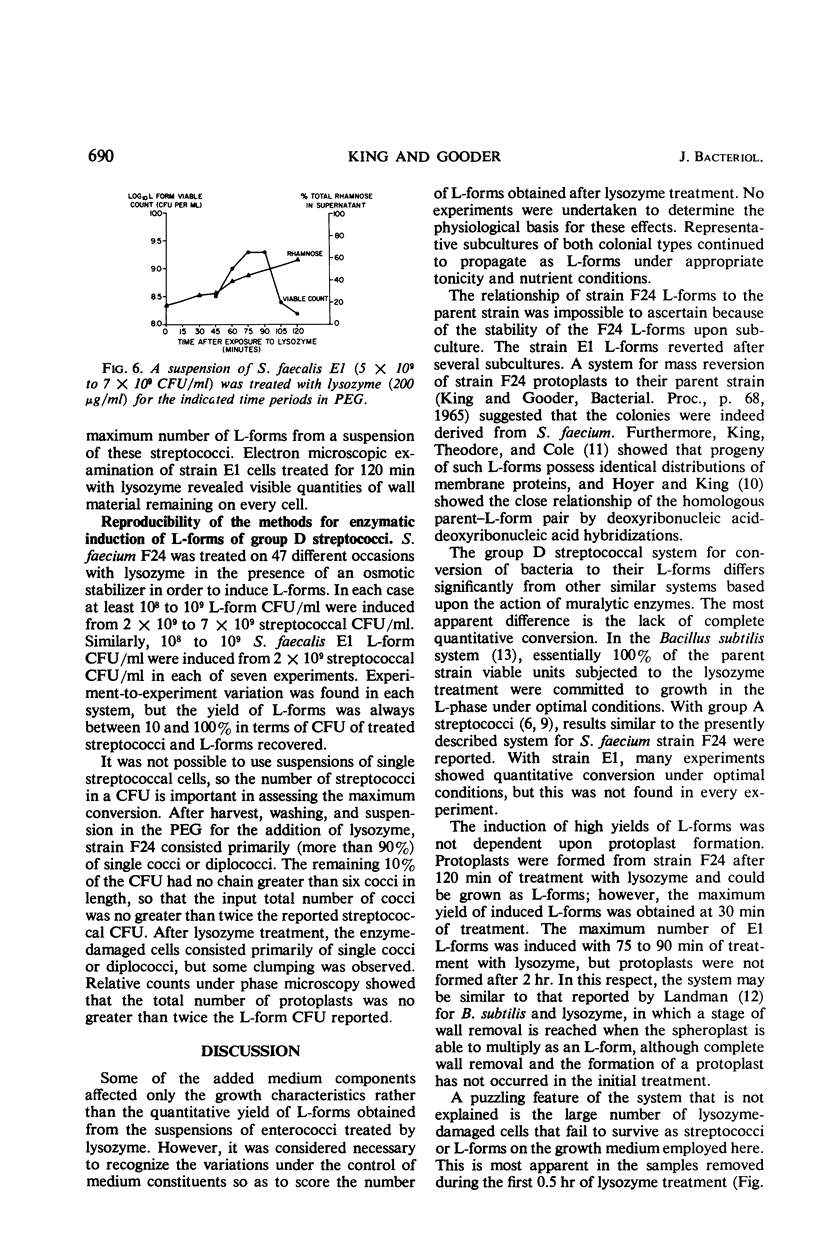
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. O-acetyl groups in the cell wall of Streptococcus faecalis. J Biol Chem. 1958 Feb;230(2):949–959. [PubMed] [Google Scholar]
- BIBB W. R., STRAUGHN W. R. Formation of protoplasts from Streptococcus faecalis by lysozyme. J Bacteriol. 1962 Nov;84:1094–1098. doi: 10.1128/jb.84.5.1094-1098.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREIMER E. H., KRAUSE R. M., McCARTY M. Studies of L forms and protoplasts of group A streptococci. I. Isolation, growth, and bacteriologic characteristics. J Exp Med. 1959 Dec 1;110:853–874. doi: 10.1084/jem.110.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer B. H., King J. R. Deoxyribonucleic acid sequence losses in a stable streptococcal L form. J Bacteriol. 1969 Mar;97(3):1516–1517. doi: 10.1128/jb.97.3.1516-1517.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. R., Theodore T. S., Cole R. M. Generic identification of L-forms by polyacrylamide gel electrophoretic comparison of extracts from parent strains and their derived L-forms. J Bacteriol. 1969 Oct;100(1):71–77. doi: 10.1128/jb.100.1.71-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDMAN O. E., HALLE S. ENZYMICALLY AND PHYSICALLY INDUCED INHERITANCE CHANGES IN BACILLUS SUBTILIS. J Mol Biol. 1963 Dec;7:721–738. doi: 10.1016/s0022-2836(63)80119-9. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., BAKAY B., CONOVER M. J., TOENNIES G. Protoplast membrane of Streptococcus faecalis. J Bacteriol. 1963 Jan;85:168–176. doi: 10.1128/jb.85.1.168-176.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Induction, colonial morphology, and growth characteristics of the L-form of Streptococcus liquefaciens. J Infect Dis. 1969 Sep;120(3):281–291. doi: 10.1093/infdis/120.3.281. [DOI] [PubMed] [Google Scholar]