Abstract
Mammographic density has been strongly associated with increased risk of breast cancer. Furthermore, density is inversely correlated with the accuracy of mammography and, therefore, a measurement of density conveys information about the difficulty of detecting cancer in a mammogram. Initial methods for assessing mammographic density were entirely subjective and qualitative; however, in the past few years methods have been developed to provide more objective and quantitative density measurements. Research is now underway to create and validate techniques for volumetric measurement of density. It is also possible to measure breast density with other imaging modalities, such as ultrasound and MRI, which do not require the use of ionizing radiation and may, therefore, be more suitable for use in young women or where it is desirable to perform measurements more frequently. In this article, the techniques for measurement of density are reviewed and some consideration is given to their strengths and limitations.
What is mammographic density?
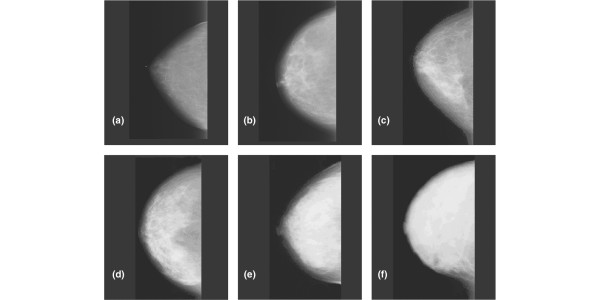
Figure 1 illustrates six mammographic images of the breast [1]. It is seen that the breast has a wide range of appearance on mammography, associated with differences in tissue composition. Radiographically the breast consists mainly of two component tissues: fibroglandular tissue and fat. Fibroglandular tissue is a mixture of fibrous connective tissue (the stroma) and the functional (or glandular) epithelial cells that line the ducts of the breast (the parenchyma). Fat has a lower X-ray attenuation coefficient (Figure 2) than fibroglandular tissue and, therefore, is more transparent to X-rays. Thus, regions of fat appear darker on a radiograph of the breast. Regions of brightness associated with fibroglandular tissue are referred to as 'mammographic density'. From the pattern of brightness in a mammographic image, the relative prevalence of these tissues in the breast can be inferred.
Figure 1.
A six-category system for classifying mammographic density. The categories describe the fraction of fibroglandular tissue in the breast as judged by an observer and are: (a) 0, (b) <10%, (c) 10–25%, (d) 26–50%, (e) 51–75%, (f) >75%. Reproduced from [1] with permission from American Association for Cancer Research.
Figure 2.
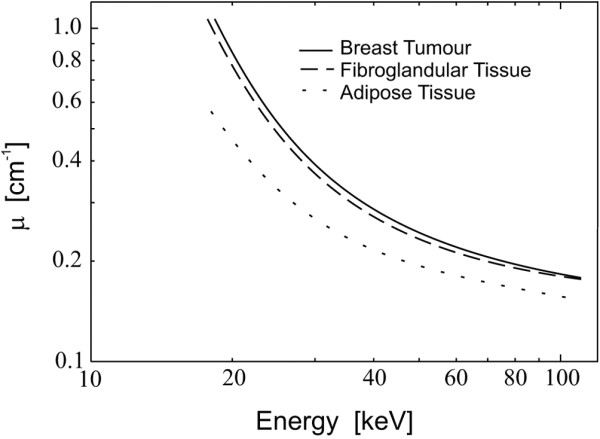
Linear X-ray attenuation coefficients of fat and fibroglandular tissue in the breast plotted versus X-ray energy. Values for samples of breast tumors are also shown. Reproduced from [51] with permission from IOP Publishing Ltd.
Parenchymal patterns and density
In 1976, John Wolfe, a radiologist who specialized in mammography, first proposed that there was a strong association between the "parenchymal patterns" seen in the mammogram and the risk that a women would later develop breast cancer [2,3]. He defined four patterns (later known as Wolfe grades) to characterize the breast. The N pattern, which represented a fatty radiolucent breast, connoted the lowest breast cancer risk. The P1 and P2 patterns indicated progressively greater levels of prominence of fibrous tissue surrounding the ducts and correspondingly higher risk, while the DY pattern indicated the highest risk with a breast that contained dense sheets of fibroglandular tissue. The association of the Wolfe patterns with risk of breast cancer has been reviewed by Saftlas and Szklo [4] and by Goodwin and Boyd [5], who concluded that there is a two- to three-fold increase in risk between the N and DY patterns. Because it appears that it is the increasing prevalence of fibroglandular tissue in the breast that gives rise to the increased risk, most subsequent work in this field has attempted to measure mammographic density explicitly.
Qualitative density assessment
n-category classification
Each of the images in Figure 1 was selected as representative of one of the categories of a six-category classification (SCC) scheme, which is quantitative for the proportion of the breast appearing as mammographically dense tissue. The six categories range from an absence of density to extensive density (the exact categories for the classification of Figure 1 are summarized in the legend).
Breast Imaging Reporting and Data System density categories
Currently, a widely used density classification scheme is the one associated with the Breast Imaging Reporting and Data System (BIRADS) [6] for reporting findings on mammography. This density system has four categories: BIRADS-1 indicates a predominantly fatty breast; BIRADS-2 scattered fibroglandular densities; BIRADS-3 a breast that is heterogeneously dense; and BIRADS-4, the highest level, an extremely dense breast that could obscure a lesion. This qualitative system was not developed to quantify risk, but to allow an interpreting radiologist to indicate the level of concern that a cancer in the breast might be missed on mammography due to masking by dense tissue. It is well known that the sensitivity of mammography is decreased in the dense breast [7,8] and a high BIRADS score tells a referring physician who is concerned about breast cancer that other tests less affected by density, such as ultrasound or magnetic resonance imaging (MRI), might be warranted. More recently, in an attempt to make the BIRADS density system more quantitative, it has been recommended that mammograms be classified into four density categories with upper bounds of 24%, 49%, 74% and 100%.
Quantitative techniques
Two-dimensional methods
Planimetry
Planimetry refers to the direct measurement of the area of dense tissue seen on the mammogram. Typically, it is performed by tracing around the regions of dense tissue on the mammogram using an instrument called a planimeter. This integrates the total enclosed area. A similar measurement of the total projected area of the breast on the mammogram is also made and the first measurement is divided by the second to obtain the fractional area of the breast that is considered to be dense. This measurement is straightforward to perform, but becomes increasingly labor intensive if it is attempted to separately measure the individual 'islands' of dense tissue that are frequently present in the image. This method was used in the work by Wolfe and colleagues [9,10].
Image digitization
For many of the quantitative density measurement techniques (but not planimetry) the image must first be digitized. Generally, this is accomplished using a device that scans the film point by point (raster scanning) or line by line with an intense, highly collimated light source such as a laser. In the digitized image, the brightness of each picture element (pixel) is represented by a numerical value ranging from 0 to 2n - 1, where n is the number of bits of digitization. This value can be either linearly or logarithmically related to the brightness. It is important that the digitizer be capable of registering signals over the full range of film opacity from clear to fully black without saturating. Generally, this requires a digitizer with at least 12 bits of precision (4,096 grey levels) combined with an optical design that supports this range. For example, the digitizer must be free from sources of extraneous glare light that would interfere with the measurement.
The digitizer must have adequate spatial resolution to allow local density changes to be tracked. For most measurements the resolution requirement is moderate and even fairly coarse digitization (for example, 0.25 mm pixels) is adequate. For more advanced measures such as texture analysis [11-13] or if the digitized image is also to be used for diagnostic purposes, it may be necessary to digitize to pixels as small as 0.050 mm (50 μm).
Thresholding
Semi-automated feature: interactive thresholding
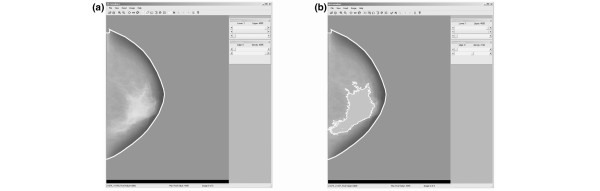
As a less time-consuming alternative to planimetry for providing a quantitative estimate of mammographically dense tissue, a simple observer-assisted technique called interactive thresholding was developed by our group [14]. This technique can easily be applied to a digital representation of the mammogram.
In the thresholding procedure, an observer manipulates a computer pointing device (for example, a mouse or trackball) to select threshold grey levels that identify specific regions of the breast. As the threshold level is adjusted, those pixels in the image at the selected level are highlighted on a color graphics overlay, so that the operator can observe interactively on the computer display when the optimal level has been set. Two threshold grey-level values are selected. The first identifies the edge of the breast to separate it from the background (area outside the breast); this threshold is referred to as iEDGE (illustrated in the breast image of Figure 3 by the dashed line). Similarly, a second threshold is selected that best outlines region(s) of mammographic density in the image, and above which all pixels are interpreted as mammographic density; this threshold is referred to as iDY (pixels of this value are represented by the solid bright line for the breast in Figure 3b). In addition, a tool is provided to allow exclusion of the area of the image of the pectoralis muscle (if it appears on the mammogram) from the calculation.
Figure 3.
The user interface for the interactive thresholding method for determination of mammographic density. (a) The digitized mammogram is displayed on the computer screen and a threshold is selected by the operator to segment the breast from the surrounding background. (b) A second threshold is set to identify the regions of density. The algorithm indicates these pixels by a white overlay.
The size of each region can be determined by counting the enclosed pixels, a process that is simplified by considering the histogram of grey-level frequencies from pixels within the breast. The histogram is constructed such that hi represents the number of pixels with grey-level i. The area under the histogram (summing all pixels in the histogram above iEDGE to the maximum grey-level iMAX) is then a measure of the projected area of the breast, A:
Pixels having a grey-level i > iDY are assumed to represent regions of mammographic density. The area under the histogram above this threshold is representative of the projected area of mammographic density in the breast. The ratio of these totals defines the proportion of mammographic density in the breast, PD:
This technique has been employed as a tool in many clinical studies [15-20].
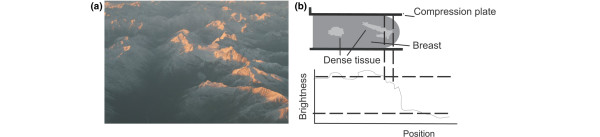
One limitation of the interactive thresholding method is that it involves operator decisions. While segmentation of the breast from the surrounding background can be performed very reproducibly, setting of the threshold to segment the dense from non-dense tissue can introduce variability. The reason for this is that there is a continuum of different signal levels in the image and a binary choice of 'dense' versus 'non-dense' and this can be complicated by local variations in thickness of the breast and in the thickness of dense tissue. An analogy is shown in Figure 4, a photograph of a mountain range. If a threshold altitude is selected to attempt to separate the snow-covered tops from the lower portions of the mountain, compromises are required. If it is attempted to include all the snow, some bare regions will be included. Conversely, if the threshold is selected to exclude all bare areas, some snow will be missed. This will impose some variability in the measurement as it does in the measurement of density. This can be minimized but not completely eliminated by training and the implementation of reading standards.
Figure 4.
Illustrates the limitations of setting a single threshold value to segment a mammogram for measurement of density. (a) Aerial view of mountains in the South Island of New Zealand. The altitude of the snow line varies so that a single value is not adequate to separate the snow-covered (dense) from bare (fatty) regions. (b) A schematic illustration of this problem. The edge and density brightness thresholds are denoted by the horizontal dashed lines Because of the reduction in thickness of the breast near the periphery, the brightness of a region of dense tissue in the mammogram (between the two vertical dashed lines) falls below the density threshold and so is excluded from the measurement. Similarly, fatty tissue in an area of the breast that is thicker than average can be inappropriately registered as dense tissue.
There have been efforts to develop automated density measurement methods based on thesholding [21-25]; however, to our knowledge, no system of this type is currently in widespread use.
Texture-based techniques
Several investigators have developed methods for analyzing mammographic patterns according to texture and found that these texture measures were associated, to varying degrees, with risk [11,26-31]. For example, Caldwell and colleagues [27] tested the correlation of the fractal dimension of the digitized mammogram with the Wolfe parenchymal patterns. Magnin and colleagues [28] in France and Giger's group at The University of Chicago [29,30] have evaluated the ability of a number of computer-calculated image texture measures to predict risk. While these ideas are intriguing and may lead to more powerful analytical tools in the future, none has yet been demonstrated to provide as strong an association with breast cancer risk as have more direct measures of mammographic density.
Volumetric density assessment
While a strong association has been demonstrated between percent mammographic density by area and breast cancer risk, it is more logical that risk is related more directly to the number of target cells, which in turn will be proportional to the 'volume' or fractional volume of dense tissue in the breast.
The most straightforward method of measuring volumetric radiological density is from computed tomography (CT). The CT scan is actually a three-dimensional reconstruction of the X-ray attenuation coefficient of tissues presented as a series of planar images. The values of each image pixel characterize the tissue in terms of its effective atomic number and electron density in a more or less continuous manner. Alternatively, if desired, a simple binary threshold can be reliably set to differentiate between fat-like and water-like tissues and the volume of each type of tissue, the total breast volume and the fraction by volume of each tissue type can be calculated.
Such data for the breast can be obtained from thoracic CT imaging performed without contrast media. There are also dedicated breast CT systems now under development in which only the breast is irradiated [32,33]. These can provide X-ray attenuation data corresponding to volume elements within the breast. One possible limitation of the latter is that because the breast is imaged when it is pendant into the imaging system with the woman lying prone on a table, some tissue near the chest wall may be excluded from the image and, therefore, from the calculation.
Another possible source of volumetric data is tomosynthesis, a technique that uses projection images obtained at different angles about the breast (Figure 5) on a specialized digital mammography system to reconstruct quasi three-dimensional planar images, essentially of the X-ray attenuation coefficient of the breast tissue [34,35]. Because only a limited number and range of angular projections are available, the reconstruction is approximate and generally will not estimate attenuation coefficients as accurately as can be done with CT. Nevertheless, the data should be more than adequate for the binary problem of having the pixels representing the tissue composition as being either fat or fibroglandular.
Figure 5.
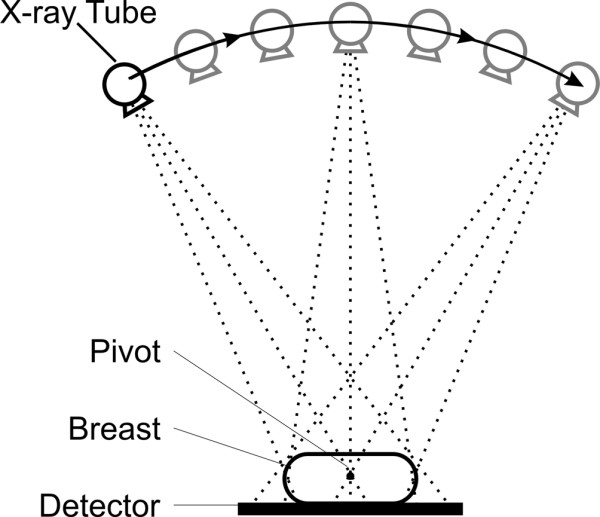
Schematic representation of image acquisition in breast tomosynthesis.
Dual-energy X-ray absorptiometry
For many years there has been strong interest in bone mineral density and, consequently, systems have been designed to measure it radiologically. Such systems are in widespread commercial use. A common approach is to make precise measurements of X-ray transmission through a defined anatomical location containing bone at two X-ray energies. If the path through a body part is assumed to consist only of bone comprising an integrated thickness of tbone, and soft tissue of integrated thickness tsoft, and the attenuation coefficients of these are known, then the transmitted fluences at the lower and higher energies are:
and
where l and h represent the low and high energies. From these two equations in two unknowns, the effective values of tbone and tsoft can be estimated. The same approach has been used for breast density measurement by Shepherd and colleagues [36], who have built a dedicated system for this purpose. Instead of bone and soft tissue, transmission through the breast is analyzed in terms of effective thicknesses of fibroglandular tissue and fat. Such a system should offer very precise results and, because of the narrow X-ray beams used, should be free of the effects of scattered radiation. One negative aspect is that even though the required radiation dose is very low, it does require a separate procedure be undertaken by the woman while most other methods simply make use of a mammogram that was obtained for other purposes.
Volumetric density from mammograms
Until three-dimensional X-ray breast imaging techniques become widely used, it is most practical to obtain volumetric density information from images produced by two-dimensional mammography systems. Several authors have suggested methods for doing this [37-39]. All methods are based on the known exponential attenuation properties of X-rays. If I0 X-rays of energy E are incident upon a breast of thickness T, with effective X-ray attenuation coefficient μ(E), then the number transmitted that can be measured by an imaging system is:
| Itr(E) = I0(E)e-μT |
This relationship is based on two simplifying assumptions: first, that the X-rays are monoenergetic; and second, that no X-rays scattered in the breast reach the imaging system.
Proceeding further, we can consider the breast to be composed of only two materials, fibroglandular tissue and fat, of thicknesses tfib and tfat such that for any path of the X-ray beam though the breast of length T:
| T = tfib + tfat |
Then
or
where is the fractional density along the measured path.
If I0/Itr is measured and T is known, then m can be calculated using the known attenuation coefficients of fibroglandular tissue and fat.
In practice, X-ray beams available for clinical mammography are polyenergetic, so the problem becomes more complicated. Researchers have taken different approaches to solve this problem. For example, Highnam and colleagues [39,40] have chosen to create a physics model of the complete image forming system, including the X-ray source, X-ray scattering and scatter removal and the image receptor, and have calculated a quantity referred to as hint, the thickness of "interesting" (that is, fibroglandular) tissue. Van Engeland and colleagues [41] developed a physical model to describe image acquisition of full-field digital mammograms and demonstrated good correlation of their volumetric density measurements with three-dimensional data from breast MRI.
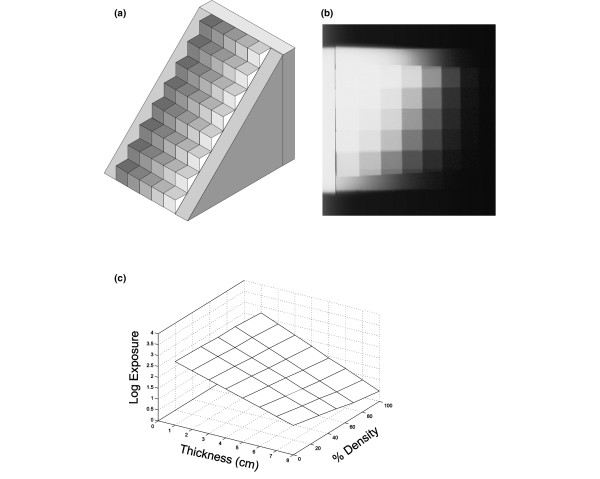
Modeling methods require good knowledge of the X-ray spectrum and all materials in the X-ray beam path from source to detector, including characterization of the performance of the antiscatter grid and detector. We have taken a slightly different, more empirical approach that avoids the need to have this specific information. We image a two-dimensional tissue equivalent 'staircase' phantom (Figure 6) varying in thickness (from 0 to 8 cm in 1 cm steps) in one dimension and in tissue composition (from pure fibro-glandular to pure fat in 8 steps) in the other. From the image of this phantom on a mammography system, acquired under a specific set of exposure factors, a surface can be determined that relates the measured attenuation to the thickness and composition of tissue represented by the steps. Then, if the breast thickness is known corresponding to each point (x, y) in the mammogram, the composition, that is, m in the last equation above, can be determined from the calibration surface.
Figure 6.
An empirical approach to calibration of a mammography system for volumetric measurement of density. (a) "Staircase" calibration tool. It is composed of a range of thicknesses of breast tissue equivalent plastics. On each step, the composition mimics fat, fibroglandular tissue and 30:70, 50:50, and 70:30 combinations of the two. (b) Radiograph of the calibration tool. (c) Calibration surface created from the radiograph in (b).
For screen-film mammography, this method is limited by the nonlinear shape of the characteristic response curve of the film. As seen in Figure 7, the response becomes very flat outside a narrow range of intensities, causing the inference of radiation exposure from the scanned measurement of film opacity (optical density) to be unreliable. To some extent this problem can be mitigated by producing calibration images at different exposure levels and bootstrapping data from these together. Nevertheless, it must be remembered that mammography was not designed to be a quantitative imaging method, but rather simply to produce an image that demonstrates lesions within the breast.
Figure 7.
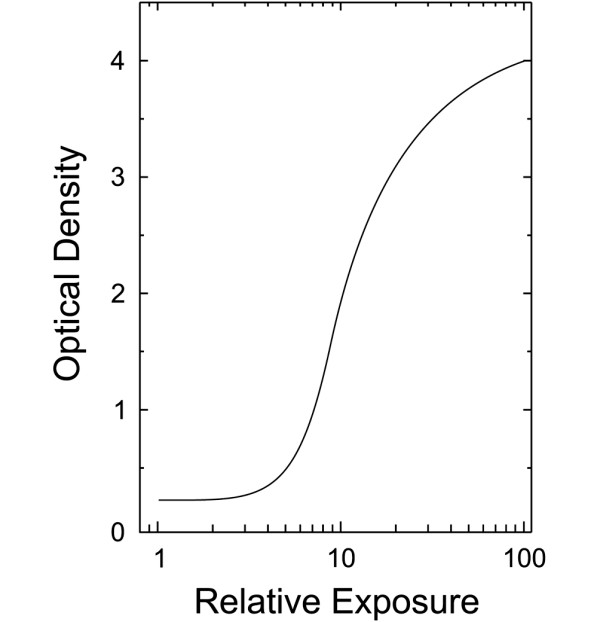
Characteristic curve of a screen-film mammography image receptor. There is an approximately linear relationship between optical density of the processed film and the logarithm of relative X-ray exposure, but only over a limited region of exposure.
Digital mammography
In digital mammography the screen-film image receptor is replaced by a detector that produces an electronic signal that precisely and predictably (with generally linear or logarithmic response) tracks the fluence of X-rays transmitted by the breast over a very wide range. This signal is digitized and the image is stored as a matrix in computer memory. This greatly facilitates quantitative density measurement, both because of the improved quality of the signal and because it is no longer necessary to scan the mammogram to digitize it. Furthermore, the modern X-ray systems used for digital mammography give highly reproducible X-ray outputs, largely eliminating the need to monitor drifting of signals from image to image.
There are a few important considerations associated with density assessment from digital mammograms. Many digital mammography systems produce images in two forms, commonly referred to as the 'for processing' or 'raw' image and the 'for presentation' or 'processed' images. The raw image data are based on the detector signal, which is normally proportional to X-ray transmission through the breast and, therefore, should relate closely to breast composition. This image would be subjected to only slight corrections, for example, to compensate for detector flaws.
In order to make the information more suitable for display on a computer screen or for laser printing on film, these images subsequently undergo extensive processing. The image processing operations may be linear or non-linear and may be applied globally (that is, over the entire image in a consistent manner) or locally. These algorithms are largely proprietary to the manufacturers of the digital mammography systems so that the exact details on what they do to the image data are not known. Such processing is likely to distort the relationship between the image signal and X-ray transmission and, thereby, interfere with the ability to derive density information from the images. Radiologists report that when viewing these processed mammograms, breasts appear to be less dense than when imaged with film mammography. For the purpose of cancer detection this is generally considered to be advantageous. In particular, attempting to measure density using thresholding algorithms or physics-based modeling algorithms is likely to be problematic, especially if such measurements are to be compared to those obtained from film mammograms.
It is strongly recommended that density analysis from digital mammograms be performed using the raw image data. Certainly volumetric analysis should be more accurate when done in this way. If it is desired to perform two-dimensional thresholding on digital mammograms, the best approach may be to transform the raw image using a clearly defined global processing algorithm that emulates the characteristics of mammography film before utilizing the thresholding algorithm to measure density. Work to evaluate the performance of density measurements made in this way is currently in progress in my laboratory.
Comparison of density assessment methods
There has been relatively little work done in comparing the measurements provided by different breast density measurement techniques and even less on comparing their performance in predicting breast cancer risk. In a study utilizing mammograms from 65 women, Martin and colleagues [42] compared two-dimensional density measurements derived from several qualitative, quantitative and semi-automated methods. These included a ten-category subjective scale based on percent density, the qualitative BIRADS scale, a newly introduced quantitative BIRADS scale (four quartiles), and a semi-automated version of a system similar to that described by Byng and colleagues [14]. Consistent with the observations of Warner and colleagues [43], they found large differences between assessments based on qualitative and quantitative methods. Qualitative assessments were also less reproducible. The authors also observed that qualitative assessments tended to overestimate the degree of density.
There have also been a limited number of comparisons between volumetric and area-based methods. While there is reason to presume that the latter should better correlate with the biological factors responsible for breast cancer risk, volumetric methods depend critically on knowledge of breast thickness, which is difficult to determine accurately in the clinical environment. This may be responsible for the recent findings that the volumetric technique developed by Highnam was less reliable than threshold-based two-dimensional thresholding [44] and did not provide a stronger predictor of breast cancer risk [45].
Density from other imaging modalities
Although most of the work on breast density measurement has been done with mammography, other medical breast imaging modalities also provide information about tissue composition. These have the advantages of providing three-dimensional images and do not involve exposure of the breast to ionizing radiation. One of these is ultrasound. Although the images primarily are sensitive to acoustic reflections at tissue boundaries, the signals are also dependent on the speed of sound and its attenuation, and all three of these factors are, in turn, dependent on tissue composition. There is indication that measurements with ultrasound could provide equivalent density information to that from mammography [46-48]. One of the current limitations of ultrasound, however, is that imaging is highly operator dependent, and this will likely lead to variability in density measurement. Nevertheless, it should be possible to produce an automated volume ultrasound system that would be reproducible and produce reliable quantitative results.
MRI images can be produced that provide signals related to the fat and water composition of the breast. Since the water composition is highly correlated with the prevalence of fibroglandular tissue, these images should be useful for density assessment. Several groups are developing approaches to quantifying density using MRI [49,50].
Conclusion
Several methods are available for the measurement of breast density. Generally, quantitative approaches that use data extracted from the digitized mammogram allow more precise and reliable measurement than possible with subjective and qualitative techniques. Methods for volumetric assessment of density are currently being developed and evaluated as well as techniques that do not require the use of ionizing radiation.
Abbreviations
BIRADS = Breast Imaging Reporting and Data System; CT = computed tomography; MRI = magnetic resonance imaging.
Competing interests
MY is a co-inventor on a patent related to the precise measurement of compressed breast thickness. This technique may become useful in quantification of breast density
Note
This article is part of a review series on Mammographic density, edited by Norman Boyd.
Other articles in the series can be found online at http://breast-cancer-research.com/articles/review-series.asp?series=bcr_Density
Acknowledgments
Acknowledgements
I would like to express my appreciation to Professor Norman Boyd, with whom I have enjoyed a collaboration over the past 18 years in the investigation of mammographic density and its connection with risk and the accuracy of breast cancer detection. Some of the work described here has been supported by funding from a Terry Fox Program Project Award through the National Cancer Institute of Canada.
References
- Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–1144. [PubMed] [Google Scholar]
- Wolfe JN. Breast patterns as an index of risk of developing breast cancer. AJR Am J Roentgenol. 1976;126:1130–1137. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::AID-CNCR2820370542>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Szklo M. Mammographic parenchymal patterns and breast cancer risk. Epidemiol Rev. 1987;9:146–174. doi: 10.1093/oxfordjournals.epirev.a036300. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Boyd NF. Mammographic parenchymal patterns and breast cancer risk: a critical appraisal of the evidence. Am J Epidemiol. 1988;127:1097–1108. doi: 10.1093/oxfordjournals.aje.a114904. [DOI] [PubMed] [Google Scholar]
- American College of Radiology . Breast Imaging Reporting and Data System (BIRADS) Reston, VA: American College of Radiology; 1993. [Google Scholar]
- Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, Geller BM, Abraham LA, Taplin SH, Dignan M, Cutter G, Ballard-Barbash R. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- Buist DS, Porter PL, Lehman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40–49 years. J Natl Cancer Inst. 2004;96:1432–1440. doi: 10.1093/jnci/djh269. [DOI] [PubMed] [Google Scholar]
- Wolfe JN, Saftlas AF, Salane M. Mammographic parenchymal patterns and quantitative evaluation of mammographic densities: a case-control study. AJR Am J Roentgenol. 1987;148:1087–1092. doi: 10.2214/ajr.148.6.1087. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Hoover RN, Brinton LA, Szklo M, Olson DR, Salane M, Wolfe JN. Mammographic densities and risk of breast cancer. Cancer. 1991;67:2833–2838. doi: 10.1002/1097-0142(19910601)67:11<2833::AID-CNCR2820671121>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Li H, Giger M, Olopade O, Margolis A, Lan L, Chinander M. Computerized texture analysis of mammographic parenchymal patterns of digitized mammograms. Acad Radiol. 2005;12:863–873. doi: 10.1016/j.acra.2005.03.069. [DOI] [PubMed] [Google Scholar]
- Miller P, Astley S. Image and Vision Computing 10. Newton, MA: Butterworth-Heinemann; 1992. Classification of breast tissue by texture analysis; pp. 277–282. [DOI] [Google Scholar]
- Megalooikonomou V, Zhang J, Kontos D, Bakic P. Analysis of texture in medical images with an application to breast imaging. In: Giger ML, Karssemeijer N, editor. Proceedings of SPIE Medical Imaging 2007: Computer-Aided Diagnosis: 20 February 2007; San Diego, CA, USA. Vol. 6514. Bellingham, WA: SPIE; 2007. p. 651421. [Google Scholar]
- Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–1638. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- Khan QJ, Kimler BF, Smith EJ, O'Dea AP, Sharma P, Fabian CJ. Correlation of mammographic breast density with Ki-67 expression in benign breast epithelial cells obtained by random periareolar fine needle aspiration of high risk women. J Clin Oncol. 2006;24:1011. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- Vachon CM, Sellers TA, Vierkant RA, Wu F-F, Brandt KR. Case-control study of increased mammographic breast density response to hormone replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11:1382–1388. [PubMed] [Google Scholar]
- Palomares MR, Machia JRB, Lehman CD, Daling JR, McTiernan A. Mammographic density correlation with Gail Model breast cancer risk estimates and component risk factors. Cancer Epidemiol Biomarkers Prev. 2006;15:1324–1330. doi: 10.1158/1055-9965.EPI-05-0689. [DOI] [PubMed] [Google Scholar]
- Mitchell G, Antoniou AC, Warren R, Peock S, Brown J, Davies R, Mattison J, Cook M, Warsi I, Evans DG, Eccles D, Douglas F, Paterson J, Hodgson S, Izatt L, Cole T, Burgess L, Eeles R, Easton DF. Mammographic density and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Res. 2006;66:1866–1872. doi: 10.1158/0008-5472.CAN-05-3368. [DOI] [PubMed] [Google Scholar]
- Gram IT, Bremnes Y, Ursin G, Maskarinec G, Bjurstam N, Lund E. Percentage density, Wolfe's and Tabár's mammographic patterns: agreement and association with risk factors for breast cancer. Breast Cancer Res. 2005;7:R854–R861. doi: 10.1186/bcr1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist DS, Aiello EJ, Miglioretti DL, White E. Mammographic breast density, dense area, and breast area differences by phase in the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2006;15:2303–2306. doi: 10.1158/1055-9965.EPI-06-0475. [DOI] [PubMed] [Google Scholar]
- Karssemeijer N. Automated classification of parenchymal patterns in mammograms. Phys Med Biol. 1998;43:365–378. doi: 10.1088/0031-9155/43/2/011. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishna R, Obuchowski NA, Chilcote WA, Powell KA. Automatic segmentation of mammographic density. Acad Radiol. 2001;8:250–256. doi: 10.1016/S1076-6332(03)80534-2. [DOI] [PubMed] [Google Scholar]
- Zhou C, Chan HP, Petrick N, Helvie MA, Goodsitt MM, Sahiner B, Hadjiiski LM. Computerized image analysis: estimation of breast density on mammograms. Med Phys. 28:1056–1069. doi: 10.1118/1.1376640. [DOI] [PubMed] [Google Scholar]
- Chang YH, Wang XH, Hardesty LA, Chang TS, Poller WR, Good WF, Gur D. Computerized assessment of tissue composition on digitized mammograms. Acad Radiol. 2002;9:899–905. doi: 10.1016/S1076-6332(03)80459-2. [DOI] [PubMed] [Google Scholar]
- Glide-Hurst CK, Duric N, Littrup P. A new method for quantitative analysis of mammographic density. Medical Physics. 2007;34:4491–4498. doi: 10.1118/1.2789407. [DOI] [PubMed] [Google Scholar]
- Taylor P, Hajnal S, Dilhuydy MH, Barreau B. Measuring image texture to separate "difficult" from "easy" mammograms. Br J Radiol. 1994;67:456–463. doi: 10.1259/0007-1285-67-797-456. [DOI] [PubMed] [Google Scholar]
- Caldwell CB, Stapleton SJ, Holdsworth DW, Jong RA, Weiser WJ, Cooke G, Yaffe MJ. Characterisation of mammographic parenchymal pattern by fractal dimension. Phys Med Biol. 1990;35:235–247. doi: 10.1088/0031-9155/35/2/004. [DOI] [PubMed] [Google Scholar]
- Magnin IE, Cluzeau F, Odet CL, Bremond A. Mammographic texture analysis: An evaluation of risk for developing breast cancer. Optical Eng. 1986;25:780–784. [Google Scholar]
- Li H, Giger ML, Huo Z, Olopade OI, Lan L, Weber BL, Bonta I. Computerized analysis of mammographic parenchymal patterns for assessing breast cancer risk: Effect of ROI size and location. Med Phys. 2004;31:549–555. doi: 10.1118/1.1644514. [DOI] [PubMed] [Google Scholar]
- Huo Z, Giger ML, Wolverton DE, Zhong W, Cumming S, Olopade OI. Computerized analysis of mammographic parenchymal patterns for breast cancer risk assessment: feature selection. Med Phys. 2000;27:4–12. doi: 10.1118/1.598851. [DOI] [PubMed] [Google Scholar]
- Li H, Giger ML, Olopade OI, Lan L. Fractal analysis of mammographic parenchymal patterns in breast cancer risk assessment. Acad Radiol. 2007;14:513–521. doi: 10.1016/j.acra.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Boone J, Nelson T, Kwan A, Yang K. TU-C-330D-03: computed tomography of the breast: design, fabrication, characterization, and initial clinical testing. Med Phys. 2006;33:2185. doi: 10.1118/1.2241505. [DOI] [Google Scholar]
- Chen B, Ning R. Cone-beam volume CT mammographic imaging: feasibility study. Med Phys. 2002;29:755–770. doi: 10.1118/1.1461843. [DOI] [PubMed] [Google Scholar]
- Niklason LT, Christian BT, Niklason LE, Kopans DB, Castleberry DE, Opsahl-Ong BH, Landberg CE, Slanetz PJ, Giardino AA, Moore R, Albagli D, DeJule MC, Fitzgerald PF, Fobare DF, Giambattista BW, Kwasnick RF, Liu J, Lubowski SJ, Possin GE, Richotte JF, Wei CY, Wirth RF. Digital tomosynthesis in breast imaging. Radiology. 1997;205:399–406. doi: 10.1148/radiology.205.2.9356620. [DOI] [PubMed] [Google Scholar]
- Wu T, Stewart A, Stanton M, McCauley T, Phillips W, Kopans DB, Moore RH, Eberhard JW, Opsahl-Ong B, Niklason L, Williams MB. Tomographic mammography using a limited number of low-dose cone-beam projection images. Med Phys. 2003;30:365–380. doi: 10.1118/1.1543934. [DOI] [PubMed] [Google Scholar]
- Shephard JA, Kerlikowske KM, Smith-Bindman R, Genant HK, Cummings SR. Measurement of breast density with dual X-ray absorptiometry: feasibility. Radiology. 2002;223:554–557. doi: 10.1148/radiol.2232010482. [DOI] [PubMed] [Google Scholar]
- Pawluczyk O, Augustine BJ, Yaffe MJ, Rico D, Yang J, Mawdsley GE, Boyd NF. A volumetric method for estimation of breast density on digitized screen-film mammograms. Med Phys. 2003;30:352–364. doi: 10.1118/1.1539038. [DOI] [PubMed] [Google Scholar]
- Kaufhold J, Thomas JA, Eberhard JW, Galbo CE, Trotter DE. A calibration approach to glandular tissue composition estimation in digital mammography. Med Phys. 2002;29:1867–1880. doi: 10.1118/1.1493215. [DOI] [PubMed] [Google Scholar]
- Highnam RP, Brady JM. Mammographic Image Processing. Boston, MA: Kluwer Academic Press; 1999. [Google Scholar]
- Highnam R, Brady M, Shepstone B. A representation for mammographic image processing. Med Image Anal. 1996;1:1–18. doi: 10.1016/S1361-8415(01)80002-5. [DOI] [PubMed] [Google Scholar]
- Van Engeland S, Snoeren PR, Huisman H, Boetes C, Karssemeijer N. Volumetric breast density estimation from full-field digital mammograms. IEEE Trans Med Imaging. 2006;25:273–282. doi: 10.1109/TMI.2005.862741. [DOI] [PubMed] [Google Scholar]
- Martin KE, Helvie MA, Zhou C, Roubidoux MA, Bailey JE, Paramagul C, Blane CE, Klein KA, Sonnad SS, Chan H-P. Mammographic density measured with quantitative computer-aided method: Comparison with radiologists' estimates and BI-RADS categories. Radiology. 2006;240:656–665. doi: 10.1148/radiol.2402041947. [DOI] [PubMed] [Google Scholar]
- Warner E, Lockwood G, Math M, Tritchler D, Boyd NF. The risk of breast cancer associated with mammographic parenchymal patterns: a meta-analysis of the published literature to examine the effect of method of classification. Cancer Detect Prev. 1992;16:67–72. [PubMed] [Google Scholar]
- McCormack VA, Highnam R, Perry N, dos Santos Silva I. Comparison of a new and existing method of mammographic density measurement: Intramethod reliability and associations with known risk factors. Cancer Epidemiol Biomarkers Prev. 2007;16:1148–1154. doi: 10.1158/1055-9965.EPI-07-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Warren R, Warsi I, Day N, Thompson D, Brady M, Tromans C, Highnam R, Easton D. Evaluating the effectiveness of using standard mammogram form to predict breast cancer risk: Case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:1074–1081. doi: 10.1158/1055-9965.EPI-07-2634. [DOI] [PubMed] [Google Scholar]
- Graham SJ, Bronskill MJ, Byng JW, Yaffe MJ, Boyd NF. Quantitative correlation of breast tissue parameters using magnetic resonance and X-ray mammography. Br J Cancer. 1996;73:162–168. doi: 10.1038/bjc.1996.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blend R, Rideout DF, Kaizer L, Shannon P, Tudor-Roberts B, Boyd NF. Parenchymal patterns of the breast defined by real time ultrasound. Eur J Cancer Prev. 1995;4:293–298. doi: 10.1097/00008469-199508000-00004. [DOI] [PubMed] [Google Scholar]
- Glide C, Duric N, Littrup P. Novel approach to evaluating breast density utilizing ultrasound tomography. Med Phys. 2007;34:744–753. doi: 10.1118/1.2428408. [DOI] [PubMed] [Google Scholar]
- Lee NA, Rusinek H, Weinreb J, Chandra R, Toth H, Singer C, Newstead G. Fatty and fibroglandular tissue volumes in the breasts of women 20–83 years old: comparison of X-ray mammography and computer-assisted MR imaging. AJR Am J Roentgenol. 1997;168:501–506. doi: 10.2214/ajr.168.2.9016235. [DOI] [PubMed] [Google Scholar]
- Klifa C, Carballido-Gamio J, Wilmes L, Laprie A, Lobo C, DeMicco E, Watkins M, Shepherd J, Gibbs J, Hylton N. IEMBS '04: 26th Annual International Conference of the IEEE: San Francisco, CA; 1–4 September 2004. Vol. 1. Piscataway, NJ: Engineering in Medicine and Biology Society; 2004. Quantification of breast tissue index from MR data using fuzzy clustering; pp. 1667–1670. [DOI] [PubMed] [Google Scholar]
- Johns PC, Yaffe MJ. X-ray characterisation of normal and neoplastic breast tissues. Phys Med Biol. 1987;32:675–695. doi: 10.1088/0031-9155/32/6/002. [DOI] [PubMed] [Google Scholar]