Abstract
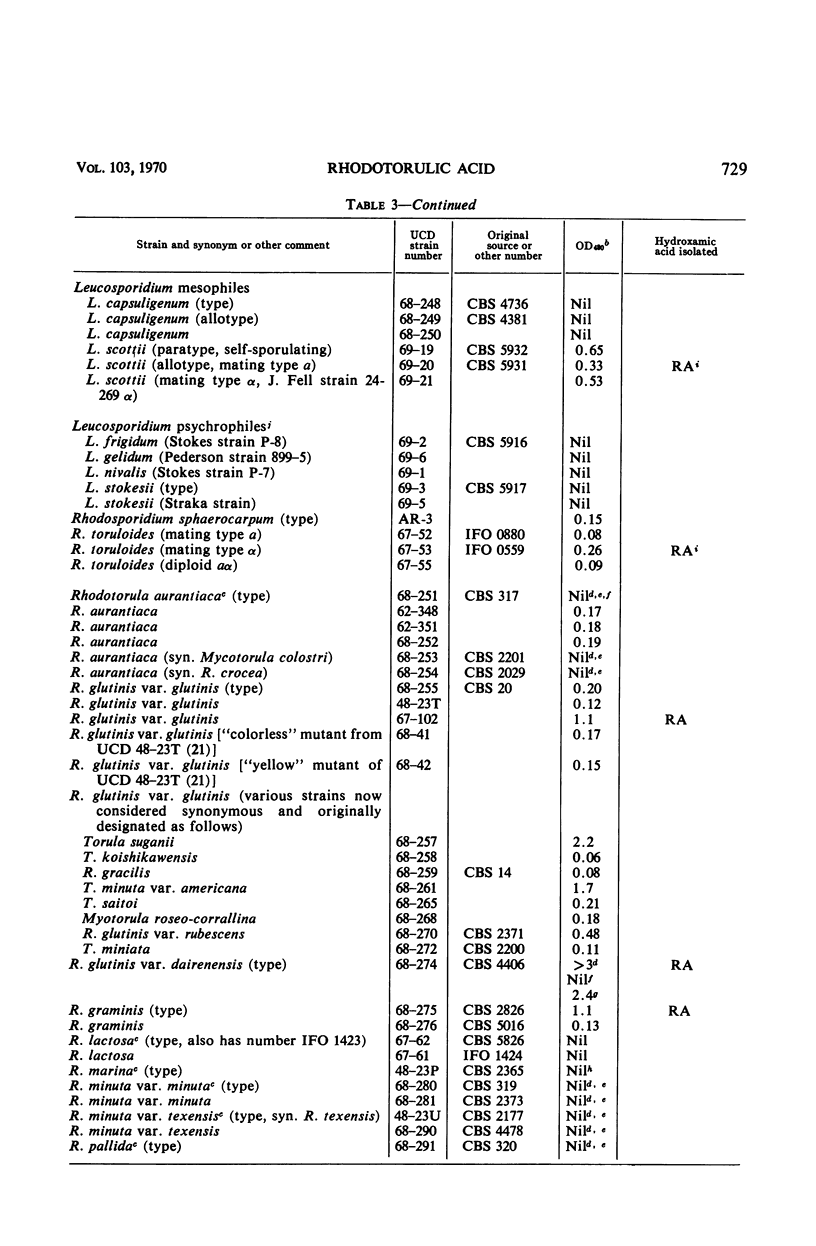
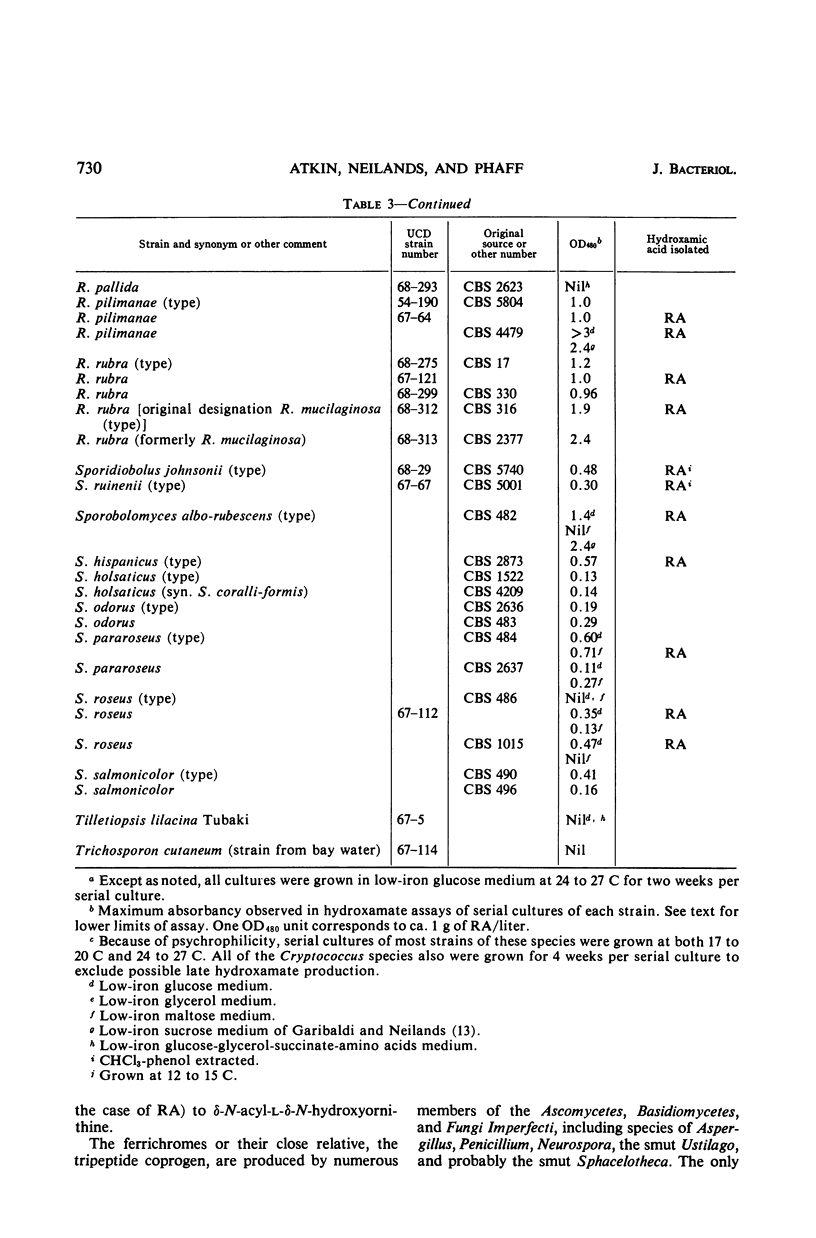
An examination of 142 strains within 19 genera of yeasts and yeastlike organisms for formation of hydroxamic acids in low-iron culture showed production of hydroxamates by two unclassified strains and by 52 strains among the genera Aessosporon (3 of 3 strains), Cryptococcus (1 of 43), Leucosporidium (3 of 11), Rhodosporidium (4 of 4), Rhodotorula (27 of 39), Sporidiobolus (2 of 2), and Sporobolomyces (12 of 13). Crystalline rhodotorulic acid was isolated in amounts sufficient to account for most or all of the measured hydroxamate in culture supernatants of 16 strains representative of the five last-mentioned hydroxamate-producing genera. A new alanine-containing ferrichrome was isolated from one strain of Cryptococcus melibiosum. Rhodotorulic acid was a major metabolic product of many of the positive strains when grown in low-iron media, and iron was shown to repress its synthesis and excretion into the culture medium. The taxonomic significance of production of hydroxamic acids is described in connection with the position of these yeast species in the subclass Heterobasidiomycetidae.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Neilands J. B. Rhodotorulic acid, a diketopiperazine dihydroxamic acid with growth-factor activity. I. Isolation and characterization. Biochemistry. 1968 Oct;7(10):3734–3739. doi: 10.1021/bi00850a054. [DOI] [PubMed] [Google Scholar]
- BICKEL H., GAEUMANN E., KELLER-SCHIERLEIN W., PRELOG V., VISCHER E., WETTSTEIN A., ZAEHNER H. [On iron-containing growth factors, sideramines, and their antagonists, the iron-containing antibiotics, sideromycins]. Experientia. 1960 Apr 15;16:129–133. doi: 10.1007/BF02157712. [DOI] [PubMed] [Google Scholar]
- BURNHAM B. F., NEILANDS J. B. Studies on the metabolic function of the ferrichrome compounds. J Biol Chem. 1961 Feb;236:554–559. [PubMed] [Google Scholar]
- BURTON M. O., SOWDEN F. J., LOCHHEAD A. G. Studies on the isolation and nature of the terregens factor. Can J Biochem Physiol. 1954 Jul;32(4):400–406. [PubMed] [Google Scholar]
- Byers B. R., Powell M. V., Lankford C. E. Iron-chelating hydroxamic acid (schizokinen) active in initiation of cell division in Bacillus megaterium. J Bacteriol. 1967 Jan;93(1):286–294. doi: 10.1128/jb.93.1.286-294.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery T. F. Initial steps in the biosynthesis of ferrichrome. Incorporation of delta-N-hydroxyornithine and delta-N-acetyl-delta-N-hydroxyornithine. Biochemistry. 1966 Nov;5(11):3694–3701. doi: 10.1021/bi00875a045. [DOI] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- HEALY W. B., CHENG S. C., McELROY W. D. Metal toxicity and iron deficiency effects on enzymes in Neurospora. Arch Biochem Biophys. 1955 Jan;54(1):206–214. doi: 10.1016/0003-9861(55)90023-0. [DOI] [PubMed] [Google Scholar]
- Kluyver A. J., van der Walt J. P., van Triet A. J. Pulcherrimin, The Pigment of Candida Pulcherrima. Proc Natl Acad Sci U S A. 1953 Jul;39(7):583–593. doi: 10.1073/pnas.39.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai H., Neilands J. B. Zinc and Cobalt: Effect on the Iron Metabolism of Ustilago sphaerogena. Science. 1966 Aug 12;153(3737):751–752. doi: 10.1126/science.153.3737.751. [DOI] [PubMed] [Google Scholar]
- Maurer B., Müller A., Keller-Schierlein W., Zähner H. Stoffwechselprodukte von Mikroorganismen. 61. Ferribactin, ein Siderochrom aus Pseudomonas fluorescens Migula. Arch Mikrobiol. 1968;60(4):326–339. [PubMed] [Google Scholar]
- Muthukrishnan S., Padmanaban G., Sarma P. S. Regulation of heme biosynthesis in Neurspora crassa. J Biol Chem. 1969 Aug 10;244(15):4241–4246. [PubMed] [Google Scholar]
- Müller A., Zähner H. Stoffwechselprodukte von Mikroorganismen. 65. Ferrioxamine aus Eubacteriales. Arch Mikrobiol. 1968;62(3):257–263. [PubMed] [Google Scholar]
- NEILANDS J. B. Some aspects of microbial iron metabolism. Bacteriol Rev. 1957 Jun;21(2):101–111. doi: 10.1128/br.21.2.101-111.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Hydroxamic acids in nature. Science. 1967 Jun 16;156(3781):1443–1447. doi: 10.1126/science.156.3781.1443. [DOI] [PubMed] [Google Scholar]
- Sayer J. M., Emery T. F. Structures of the naturally occurring hydroxamic acids, fusarinines A and B. Biochemistry. 1968 Jan;7(1):184–190. doi: 10.1021/bi00841a023. [DOI] [PubMed] [Google Scholar]
- Slodki M. E., Wickerham L. J., Bandoni R. J. Extracellular heteropolysaccharides from Cryptococcus and Tremella. A possible taxonomic relationship. Can J Microbiol. 1966 Jun;12(3):489–494. doi: 10.1139/m66-071. [DOI] [PubMed] [Google Scholar]
- Snow G. A., White A. J. Chemical and biological properties of mycobactins isolated from various mycobacteria. Biochem J. 1969 Dec;115(5):1031–1050. doi: 10.1042/bj1151031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKERHAM L. J., BURTON K. A. Hybridization studies involving Saccharomyces fragilis and Zygosaccharomyces dobzhanskii. J Bacteriol. 1956 Mar;71(3):296–302. doi: 10.1128/jb.71.3.296-302.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKERHAM L. J., BURTON K. A. Hybridization studies involving Saccharomyces lactis and Zygosaccharomyces ashbyi. J Bacteriol. 1956 Mar;71(3):290–295. doi: 10.1128/jb.71.3.290-295.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Knüsel F. Permeability of Staphylococcus aureus to the Sideromycin antibiotic A 22,765. Arch Mikrobiol. 1969 Oct;68(2):107–112. doi: 10.1007/BF00413870. [DOI] [PubMed] [Google Scholar]


