Abstract
Mutational analysis has established that the cytoplasmic tail of the integrin β3 subunit binds c-Src (termed as Src in this study) and is critical for bidirectional integrin signaling. Here we show in washed human platelets that a cell-permeable, myristoylated RGT peptide (myr-RGT) corresponding to the integrin β3 C-terminal sequence dose-dependently inhibited stable platelet adhesion and spreading on immobilized fibrinogen, and fibrin clot retraction as well. Myr-RGT also inhibited the aggregation-dependent platelet secretion and secretion-dependent second wave of platelet aggregation induced by adenosine diphosphate, ristocetin, or thrombin. Thus, myr-RGT inhibited integrin outside-in signaling. In contrast, myr-RGT had no inhibitory effect on adenosine diphosphate-induced soluble fibrinogen binding to platelets that is dependent on integrin inside-out signaling. Furthermore, the RGT peptide induced dissociation of Src from integrin β3 and dose-dependently inhibited the purified recombinant β3 cytoplasmic domain binding to Src-SH3. In addition, phosphorylation of the β3 cytoplasmic tyrosines, Y747 and Y759, was inhibited by myr-RGT. These data indicate an important role for β3-Src interaction in outside-in signaling. Thus, in intact human platelets, disruption of the association of Src with β3 and selective blockade of integrin αIIbβ3 outside-in signaling by myr-RGT suggest a potential new antithrombotic strategy.
Introduction
Integrins mediate cell adhesion and transduce signals that are critical in the dynamic regulation of cell adhesion, spreading, migration, and proliferation.1,2 Bidirectional signaling of integrins is exemplified in the prototype platelet integrin, αIIbβ3. Inside-out signaling of integrin αIIbβ3 requires talin binding to the cytoplasmic domain of αIIbβ3,3 and its subsequent conformational changes,4 which propagate to the extracellular ligand binding domain, activate the ligand binding function. Ligand binding to αIIbβ3 not only forms adhesive bonds but also induces outside-in signaling, leading to platelet spreading, secretion, amplification of platelet aggregation, and subsequent clot retraction.5 It is known that outside-in signaling of integrin αIIbβ3 requires phosphorylation of the β3 cytoplasmic domain at Y747 and Y759.6 It has also been reported, using multiple Src family protein tyrosine kinase-deficient mouse models, that Src plays an important role in the integrin outside-in signaling.7 However, the exact molecular events and the direct requirement for this kinase in β3 tyrosine phosphorylation in human platelets remain to be established
The cytoplasmic domain of β3 is critical in integrin bidirectional signaling. Inside-out signaling requires the membrane proximal region of β3, including the highly conserved N744PXY747 motif, which directly interacts with the talin head domain, allowing receptor activation. Arias-Salgado et al have shown that the C-terminus of β3 interacts with Src, which is important in integrin outside-in signaling leading to cell spreading, and that 2 residues in the β3 cytoplasmic tail, R760 and T762, are necessary for Src binding.8 Furthermore, deletion of the C-terminal RGT sequence of β3 abolishes Src binding.9 However, whether RGT sequence is sufficient for Src binding or whether additional residues upstream of R760, in particular Y759 of the NXXY motif, are also involved in this interaction, is still an unanswered question. Using a CHO cell model, we have previously identified the β3 C-terminal end, in particular the last 3 R760GT762 residues, as an important functional domain necessary for outside-in signaling leading to αIIbβ3-dependent cell spreading but dispensable for inside-out signaling.10 More recently, we have shown that calpain cleavage of the β3 cytoplasmic tail, which preferentially occurs at Y759 removing the RGT residues, is prevented by phosphorylation of the β3 cytoplasmic tail residues Y747 and Y759.11 These results suggested a protective effect of tyrosine phosphorylation, in which Src is presumably involved, on β3 cytoplasmic tail cleavage. However, in human platelets, the precise functional role of the R760GT762 residues in outside-in signaling remains to be established.
Much of our knowledge with respect to the physiologic and pathologic consequences of integrin outside-in signaling in platelets was obtained with genetically manipulated mice in which this critical signaling pathway is disrupted. Compared with the integrin β3-deficient mice, which show all the cardinal features of the human bleeding disorder Glanzmann thrombasthenia, transgenic mice with selectively impaired outside-in signaling all presented a common phenotype characterized by unstable thrombus formation but a normal or only slightly prolonged bleeding time.6,12–20 Outside-in signaling in platelets thus appears to be mainly responsible for thrombus stabilization, and selective interference with the propagation of outside-in signals might represent a new therapeutic approach to selectively inhibit platelet-rich arterial thrombosis with less bleeding complications caused by compromised primary hemostasis. Because human platelets are not amenable to genetic manipulations, we chose to develop a cell-permeable RGT peptide to investigate its effect on αIIbβ3-dependent outside-in signaling. Our results show that a myristoylated RGT peptide was readily internalized into human platelets and did indeed dose-dependently and selectively interfere with integrin outside-in signaling in platelets as indicated by its inhibitory effects on platelet spreading, secretion, and clot retraction mediated by washed human platelets. Importantly, myristoylated RGT (myr-RGT) abolished β3 interaction with Src SH3 domain and inhibited β3 cytoplasmic tyrosine phosphorylation. Thus, Src binding to β3 cytoplasmic domain is required for Src-dependent phosphorylation of β3 tyrosine residues, and myr-RGT may potentially be developed into a new type of antithrombotic agent.
Methods
This study was approved by the Institutional Review Board of Shanghai Jiaotong University School of Medicine.
Antibodies and reagents
Monoclonal antibodies to integrin β3 (SZ21), αIIb (SZ22), and fluorescein isothiocyanate (FITC)-anti-CD62P were from Beckman Coulter (Fullerton, CA), antitalin (8d4) from Sigma-Aldrich (St Louis, MO), anti-glutathione-S-transferase (GST) (B14) from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-His (AB102) from Tiangen (Beijing, China). Monoclonal antibody 327 against c-Src SH3 domain and polyclonal rabbit antibodies specific for human integrin β3 phospho-Y747 or phospho-Y759 were products of Abcam (Cambridge, MA). Horseradish peroxidase- or FITC-conjugated secondary antibodies were from Biosource International (Camarillo, CA). Ristocetin was purchased from Sigma-Aldrich and RGDS peptide from Bachem California (Torrance, CA). Alexa Fluor 488-conjugated human fibrinogen was a product of Invitrogen (Carlsbad, CA). Purified human fibrinogen and human α-thrombin were purchased from Enzyme Research Laboratories (South Bend, IN) and protein G plus-Agarose from Santa Cruz Biotechnology. All other reagents were obtained from Sigma-Aldrich.
Peptides
The synthetic peptides were obtained from GL Biochem (Shanghai, China), and myristoylated peptides were synthesized with myristoylate covalently linked to their N-terminal amino acid. The peptides were purified by reverse phase-high performance liquid chromatography using a C-18 column with a purity higher than 98%. The expected mass spectra were verified by electrospray ion-trap mass spectrometry. To test the peptides for their ability to penetrate the membrane of living cells, myristoylated and nonmyristoylated RGT peptides were labeled with FITC (Sigma-Aldrich) as described21 and assayed by flow cytometry and confocal microscopy.
Plasmid constructs
The cDNAs encoding the SH3 domain of human c-Src and the wild-type or mutant integrin β3 cytoplasmic tail were obtained by polymerase chain reaction amplification and cloned into the pGEX-6P1 vector (GE Healthcare, Little Chalfont, United Kingdom) downstream of the GST sequence. The Src-SH3 cDNA was also inserted into the pET32A vector (Novagen, Madison, WI). All constructs were verified by DNA sequencing.
Platelet preparation22
Whole blood was drawn by venipuncture from healthy volunteers with informed consent, and collected into 1/10th volume of 3.8% (w/v) trisodium citrate. The blood was centrifuged at 300g for 20 minutes at 22°C, and platelet-rich plasma (PRP) was collected. Platelet counts in PRP were adjusted to 3 × 108/mL by adding platelet-poor plasma. For preparation of washed platelets, blood was anticoagulated by 1/7 volume of acid citrate dextrose (ACD; 85 mM of trisodium citrate, 83 mM of dextrose, and 21 mM of citric acid). The PRP was recentrifuged at 1500g for 20 minutes. Pelleted platelets were washed twice with CGS (120 mM of NaCl, 13 mM of trisodium citrate, 30 mM of glucose, pH 6.5), and were finally resuspended at a concentration of 3 × 108/mL in Tyrode's buffer (137 mM of NaCl, 2 mM of KCl, 12 mM of NaHCO3, 0.3 mM of NaH2PO4, 5.5 mM of glucose, 5 mM of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 0.1% bovine serum albumin (BSA), pH 7.4) containing extemporaneously added 1 mM of CaCl2 and MgCl2. The platelet suspension was allowed to stand at room temperature for 60 minutes before use.
Platelet adhesion assay
The platelet adhesion assay was performed as previously described with minor modifications.10,23 In brief, 96-well microtiter plates were coated overnight at 4°C with either human fibrinogen (20 μg/mL) or BSA (10 mg/mL) in 0.1 M of NaHCO3, pH 8.3. The wells were blocked for 2 hours at room temperature with 20 mg/mL of BSA. Washed platelets in Tyrode's buffer at a concentration of 2 × 108/mL were incubated with peptides at indicated concentrations for 30 minutes at 37°C. Platelets (100 μL) were added to the precoated wells in triplicate and incubated at 37°C for 60 minutes. Nonadherent platelets were removed by 3 vigorous washes with phosphate-buffered saline, and adherent platelets were quantified by a phosphatase assay. In another set of experiments, the platelets were incubated with the peptide, the peptide-containing supernatant was removed by centrifugation, and the peptide-treated platelets were resuspended in Tyrode's buffer for adherent assay.24 In addition, to monitor peptide-dependent platelet lysis, the platelet phosphatase activity was separately measured in the supernatant of the platelets in suspension or after their adhesion to fibrinogen by mixing with p-nitrophenyl phosphatase (PNPP) substrate solution (100 mM of sodium acetate, 1% Triton X-100, 3 mg/mL of PNPP) for 60 minutes at 37°C. The enzyme reaction was stopped with 1 M of NaOH, and the optical density was measured at 405 nm with a microplate reader.
Platelet spreading on immobilized fibrinogen
Platelet spreading assays10 were performed using the Lab-Tek chamber slides (Nalge Nunc International, Rochester, NY) that were precoated with 20 μg/mL of fibrinogen. Platelets (50 μL) at 1 × 108 cells/mL in Tyrode's buffer were incubated for 30 minutes with the peptides, then transferred to the Lab-Tek wells and allowed to adhere for 60 minutes at 37°C. After washing, the adherent platelets were fixed with 1% paraformaldehyde, followed by an incubation with 1% BSA. Platelets were stained with a monoclonal antibody against human integrin β3 and an FITC-conjugated goat antimouse immunoglobulin antibody. Coverslips were mounted on the slides with Dako fluorescent mounting medium (Dako North America, Carpinteria, CA), and the adherent platelets were examined by confocal microscopy using an LSM510 confocal microscope (Zeiss, Jena, Germany) with a 63× plan-apochromat differential interference contrast (DIC) oil- immersion objective. The images were processed with LSM 5 Pascal software (Zeiss).
Clot retraction
Clot retraction was performed as described previously.25,26 In brief, washed platelets (3 × 108/mL) in resuspension buffer (10 mM of HEPES, pH 7.4, 140 mM of NaCl, 3 mM of KCl, 0.5 mM of MgCl2, 5 mM of NaHCO3, 10 mM of glucose) with extemporaneously added CaCl2 (1 mM) were incubated with the peptides for 30 minutes at 37°C. Fibrinogen was then added to a final concentration of 2 mg/mL, and the platelets were dispensed in 0.25-mL aliquots into siliconized glass tubes. Clot retraction was initiated by the addition of human α-thrombin to a final concentration of 1 U/mL and allowed to proceed at 37°C. Clot retraction was monitored by taking photographs at indicated time points using a digital camera. Clot retraction was quantified on the photographs by measuring the clot surface area with the NIH Image 1.67e software, and the data were processed using Excel 4.0. Results were expressed as percentage of retraction (% = area t/area t0 × 100%).
Platelet aggregation
Platelet aggregation was performed as previously described.22,27 PRP at a platelet concentration of 3 × 108/mL was preincubated in glass vials for 10 minutes at 37°C in the presence or absence of peptides and stimulated with adenosine diphosphate (ADP) (2 μM), ristocetin (1.25 mg/mL), or thrombin receptor activating peptide (TRAP) (10 μM) at 37°C under constant stirring at 1000 rpm. Thrombin-induced platelet aggregation was performed with washed platelets at a concentration of 2.5 × 108/mL in Tyrode's buffer and by the addition of thrombin (0.1 U/mL). Platelet aggregation was monitored in a lumi-aggregometer (Chrono-Log, Havertown, PA).
Soluble fibrinogen binding
Washed platelets were suspended in Tyrode's buffer at 3 × 108/mL, and fibrinogen binding was measured according to methods described elsewhere.28,29 Peptides were added to the platelets up to 250 μM for a 30-minute incubation at 37°C. Then the suspension was incubated with or without ADP (20 μM) in the presence of Alexa Fluor 488-labeled fibrinogen (100 μg/mL) for 30 minutes at room temperature in dark. Platelet-bound fluorescence was analyzed on an EPICS XL flow cytometer (Beckman Coulter). Samples treated with 1 mM of RGDS served as control.
P-selectin expression
P-selectin expression was measured with a protocol as described elsewhere30 using fluorescein-labeled anti-CD62P antibody. Washed platelets in Tyrode's buffer at a concentration of 2 × 108/mL were preincubated with peptides (250 μM) at 37°C for 30 minutes. The platelets were then stimulated for 3 minutes with or without thrombin (0.1 U/mL) at 37°C and immediately fixed with 1% paraformaldehyde. The fixed platelets were labeled with an FITC-CD62P antibody at concentrations recommended by the manufacturers. A same conjugated nonspecific mouse isotype IgG was used as negative control. P-selectin surface expression was analyzed by flow cytometry. P-selectin expression was also analyzed in peptide-treated platelets stimulated with ADP (20 μM) under stirring for 3 minutes in an aggregometer.
Tyrosine phosphorylation of integrin β3 tail in platelets
Tyrosine phosphorylation of the integrin β3 cytoplasmic tail was estimated by Western blot.11,31 Washed platelets (1 × 109/mL in Tyrode's buffer) were preincubated with peptides at 250 μM for 30 minutes and were stimulated by α-thrombin (0.05 U/mL) with stirring at 1000 rpm for 1 minute. The reaction was terminated by the addition of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 1 mM of ethylenediaminetetraacetic acid (EDTA), 2 mM of sodium vanadate, 1 mM of phenylmethylsulfonyl fluoride, complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Samples were subjected to SDS-PAGE analysis using 8% gels and immunoblotted with various antibodies. Enhanced chemiluminescence was used for signal detection (Pierce Chemical, Rockford, IL).
Immunoprecipitation
Experiments were performed according to the procedures published elsewhere.32,33 Platelets in suspension at 2 to 4 × 108/mL were preincubated with peptides (250 μM) for 30 minutes at 37°C, and lysates were prepared by solubilizing the platelets with cold Nonidet P-40 lysis buffer (1% NP-40, 150 mM of NaCl, 50 mM of Tris, pH 7.2, 1 mM of EDTA, 1 mM of sodium vanadate, 1 mM of phenylmethylsulfonyl fluoride) containing the protease inhibitor mixture. Platelet lysates (600 μg protein) were incubated with a monoclonal antibody against either integrin β3 or Src for 1 hour at 4°C with gentle agitation and were further incubated with protein G-Agarose beads (20 μL) overnight at 4°C. After washing, immunoprecipitates were resuspended in SDS sample buffer containing 5% 2-mercaptoethanol and subjected to SDS-PAGE analysis using 10% gels. Src, integrin αIIb or β3 subunits were identified by Western blotting using specific monoclonal antibodies.
Enzyme-linked immunosorbent assay
Peptides were coated to the wells of microtiter plates at a concentration of 20 μg/mL. The SH3 domain of c-Src was expressed as a GST fusion protein in Escherichia coli strain BL21 (Novagen) and purified from bacterial extracts.32 Increasing amounts (2.5–20 μM) of purified GST-Src-SH3 (in 100 μL) were added to the wells in triplicate and incubated for 1 hour at room temperature. After subsequent incubations with monoclonal anti-GST antibody and horseradish peroxidase-conjugated antimouse immunoglobulin, color was developed with o-phenylenediamine substrate. Absorbance was measured in an enzyme-linked immunosorbent assay reader at 450 nm.
Pull-down assays
Wild-type or mutated GST-β3 cytoplasmic tail fusion proteins (GST-β3, GST-β3Y/A, GST-β3-741) were expressed in E coli and purified from bacterial lysates by batch elution from glutathione-Sepharose. Purified GST fusion proteins coupled to glutathione-Sepharose 4B beads were incubated overnight at 4°C with platelet lysates in lysis buffer (50 mM of Tris, pH 7.4, 75 mM of NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 5 mM of EDTA, 1 mM of sodium vanadate and the protease inhibitor mixture) in the presence or absence of the peptide, and immunoblotted with specific antibodies. His-tagged Src-SH3 was expressed in BL21 (DE3) and purified by Ni-affinity chromatography. For β3-Src-SH3 pull-down assays, immobilized wild-type GST-β3 was incubated with His-Src-SH3 in the presence of increasing concentrations of the RGT peptide overnight at 4°C under constant rotation. Complexes were washed and subjected to Western blot analysis.
Results
Membrane permeability of the myr-RGT peptide
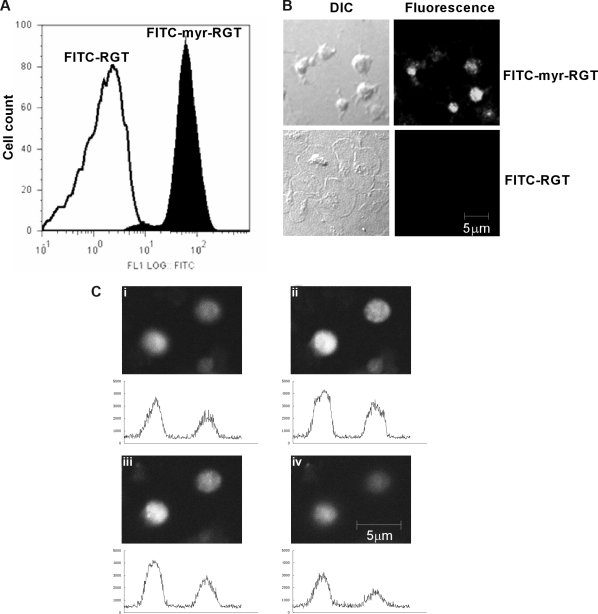
Peptides were labeled with FITC to trace their localization. Flow cytometry analysis showed that platelets incubated with FITC-labeled myr-RGT became fluorescent (Figure 1A). In confocal microscopy analysis, all platelets treated with myr-RGT exhibited a strong overall fluorescence (Figure 1B). In contrast, platelets treated with the nonmyristoylated peptide, although visible with DIC imaging, were fluorescently negative (Figure 1B). Z-position sectional analysis of FITC-myr-RGT-treated platelets confirmed the intracellular localization of the peptide (Figure 1C).
Figure 1.
Intraplatelet localization of the membrane-permeable peptides. Platelets incubated with FITC-conjugated peptides (250 μM) for 30 minutes and analyzed by flow cytometry and fluorescence microscopy. (A) Fluorescence histograms of platelets treated with FITC-conjugated myristoylated RGT peptide (FITC-myr-RGT, closed histogram) or with FITC-conjugated RGT peptide (FITC-RGT, open histogram) were analyzed by flow cytometry. (B) Platelets were treated with FITC-myr-RGT peptide or FITC-RGT peptide and allowed to spread on immobilized fibrinogen for 60 minutes. The same microscopic fields were analyzed by differential interference contrast (DIC) microscopy as well as confocal fluorescence microscopy (fluorescence). Figure shows representative images made by a Zeiss LSM510 confocal microscope with a 63× plan-apochromat DIC oil-immersion objective with Pascal software. (C) Z-Stack scanning was performed on FITC-myr-RGT-treated platelets with intervals of 1.2 μm (from 1 to 4). The fluorescence density profiles are shown below each picture.
Inhibition of platelet stable adhesion and spreading on immobilized fibrinogen by myr-RGT
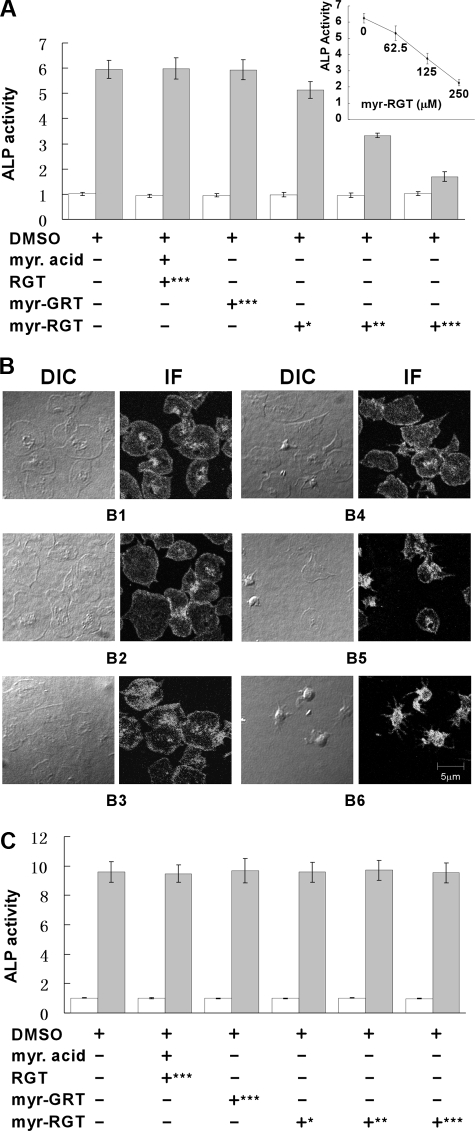
An important function of integrin αIIbβ3 outside-in signaling is to mediate stable platelet adhesion and spreading on immobilized ligands. Platelets with impaired outside-in signaling are hence less resistant to detachment in adhesion assays. For quantitative analysis of the effect of the RGT peptide on platelet adhesion, we used a phosphatase assay. As shown in Figure 2A, myr-RGT dose-dependently inhibited platelet stable adhesion on immobilized fibrinogen, whereas control peptides, or myristic acid or the vehicle dimethyl sulfoxide (DMSO), had no significant inhibitory effect. A 50% inhibition of platelet adhesion was obtained with a 125 μM concentration of myr-RGT, whereas maximal inhibi-tion was achieved at 250 μM. Removal of myr-RGT from the adhesion buffer did not significantly alter the adhesion of myr-RGT-pretreated platelets (Figure 2A inset). Parallel analysis of DIC images revealed a dose-dependent inhibition of platelet spreading on immobilized fibrinogen by myr-RGT (Figure 2B). In addition, we measured the phosphatase activity in the supernatant of adherent platelets or of platelets in suspension. No difference was observed between myr-RGT-treated and nontreated platelets either after adhesion (Figure 2A) or in suspension (Figure 2C), indicating that myr-RGT did not induce platelet lysis.
Figure 2.
Quantitative analysis of the effect of myristoylated RGT peptide on platelet stable adhesion and spreading on immobilized fibrinogen. (A) Platelets were added to microtiter wells precoated with fibrinogen and allowed to adhere for 60 minutes at 37°C. The phosphatase activity in supernatants (open columns) or in adherent platelets (closed columns) was quantified by a PNPP assay. Data of 3 experiments (mean ± SD) were presented as the ratio of the phosphatase activity of platelet samples over blank. Peptide concentrations: *62.5 μM; **125 μM; ***250 μM. (Inset) The phosphatase activity of myr-RGT-treated platelets adherent to immobilized fibrinogen after removal of the peptide from the buffer. (B) Microphotographs of platelets adherent on fibrinogen and treated with DMSO (B1), myristic acid and RGT peptide at a concentration of 250 μM (B2), scrambled myr-GRT at 250 μM (B3), myr-RGT at 62.5 μM (B4), 125 μM (B5), and 250 μM (B6). DIC indicates differential interference contrast microscopy; IF: immunofluorescence assay with anti-integrin β3 antibody. (C) Platelets were incubated in suspension with different treatments as indicated for 30 minutes at 37°C. The phosphatase activity in supernatants (□) or in remaining platelet suspensions ( ) was quantified by a PNPP assay. Data are arranged as in panel A.
) was quantified by a PNPP assay. Data are arranged as in panel A.
Effect of myr-RGT on platelet-mediated fibrin clot retraction
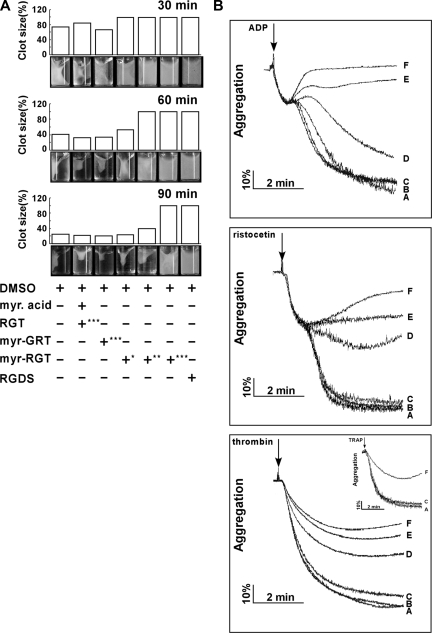
Platelet clot retraction is an essential step in platelet thrombus consolidation and is initiated after platelet activation as a late consequence of integrin outside-in signaling. It also relies on an efficient extracellular fibrinogen-integrin interaction as well as a stable integrin-cytoskeletal association. To investigate whether myr-RGT has an effect on this important platelet function, we performed a plasma-free platelet clot retraction assay using washed platelets pretreated with different peptides. Fibrin clots were induced at 37°C by the addition of thrombin to platelet suspension containing purified fibrinogen, and the subsequent platelet-dependent clot retraction was monitored over a 90-minute time period. As shown in Figure 3A, at 30, 60, and 90 minutes, the fibrin clots in the control tubes underwent a time-dependent retraction that started at 30 minutes and was complete at 90 minutes. For quantitative analyses of the effects of the different peptides, clot retraction was measured at 60 minutes. The presence of myr-RGT at 62.5 μM resulted in a weak, but recognizable, inhibition of clot retraction. When the concentration of the peptide was increased to 125 μM, the inhibition reached a full scale in terms of clot size, whereas the clot retraction did occur under this condition to a subtle extent, judging from the change of clot density. A complete inhibition was achieved with the addition of 250 μM of myr-RGT, a result comparable with that seen in the presence of 1 mM RGDS known to inhibit clot retraction by preventing the extracellular fibrinogen-integrin αIIbβ3 interaction. These results demonstrated that myr-RGT dose-dependently inhibited clot retraction in an experimental system with purified fibrinogen and washed platelets.
Figure 3.
Effect of myr-RGT on fibrin clot retraction and platelet aggregation. (A) Washed platelets were resuspended in HEPES buffer and incubated with different peptides or their vehicles as indicated. Then 2 mg/mL of human fibrinogen was added and fibrin clot formation was initiated by adding 1 U/mL of thrombin. Clot retraction was monitored over time, and photographs of the clots were taken at different time points (bottom panel). Peptide concentrations: *62.5 μM; **125 μM; ***250 μM. The histograms of the clot size were generated from the photographs by calculating the ratio of the surface area of the retracted clot versus that of the initial clot. (B) Aggregation of nontreated or peptide-treated platelets was induced in an aggregometer at 37°C under constant stirring (1000 rpm) by ADP (2 μM), ristocetin (1.25 mg/mL) in PRP, or by thrombin (0.1 U/mL) in washed platelets preincubated with (A) DMSO, (B) myristic acid and RGT peptide at a concentration of 250 μM, (C) scrambled myr-GRT at 250 μM, myr-RGT at concentrations of (D) 62.5 μM, (E) 125 μM, and (F) 250 μM, respectively. (Inset) Platelets in plasma treated with 250 μM of (F) myr-RGT, (C) scrambled myr-GRT, or (A) vehicle DMSO were stimulated by TRAP (10 μM) to aggregate.
Modulation of platelet aggregation by myr-RGT
Full-scale platelet aggregation in response to low concentrations of agonists comprises the first wave of reversible aggregation that is mediated by inside-out signaling and ligand binding to αIIbβ3, the second wave of irreversible aggregation that requires αIIbβ3-dependent outside-in signaling, and consequent aggregation-dependent platelet granule secretion.5 To determine whether myr-RGT interferes with integrin signaling during platelet activation, we investigated its effect on platelet aggregation induced by ADP and ristocetin. It is notable, as shown in Figure 3B, that in the presence of myr-RGT, the primary wave of platelet aggregation was intact. In contrast, the secondary wave of platelet aggregation was significantly affected at a peptide concentration of 125 μM and was completely abrogated at higher concentrations (250 μM). We also tested the effect of myr-RGT on washed platelet aggregation induced by low-dose thrombin. In the presence of myr-RGT at 62.5 μM, 125 μM, or 250 μM, the aggregation in response to thrombin was reduced to 60%, 46%, and 39% of controls, respectively. In control experiments, myristic acid plus equal concentrations of nonmyristoylated RGT peptide did not inhibit aggregation. Furthermore, the scrambled peptide, myr-GRT, failed to significantly affect platelet aggregation, even at a high concentration (250 μM). In addition, 250 μM of myr-RGT inhibited TRAP-induced aggregation (Figure 3B inset).
Effect of myr-RGT on soluble fibrinogen binding
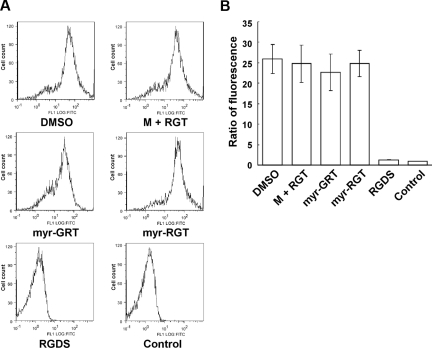
To investigate whether myr-RGT affects inside-out integrin signaling, we examined its effect on soluble fibrinogen binding to integrin αIIbβ3, the method of choice to assess integrin αIIbβ3 activation after inside-out signaling. Interestingly, as shown in Figure 4, when stimulated by ADP, platelets preincubated with myr-RGT demonstrated an equivalent ability to bind soluble Alexa Fluor 488-conjugated fibrinogen as those preincubated with the control scrambled peptide or the nonmyristoylated RGT peptide at concentrations up to 250 μM. In contrast, soluble fibrinogen binding to ADP-stimulated platelets was almost completely inhibited by 1 mM of the integrin inhibitor RGDS (Figure 4B). These data provide evidence that inside-out signaling and thus the ligand binding to the integrin αIIbβ3 was not affected by the intracellular presence of myr-RGT.
Figure 4.
Effect of myr-RGT on soluble fibrinogen binding to platelets. Platelets were preincubated with different peptides or their vehicle, and binding of Alexa Fluor 488-conjugated fibrinogen (100 μg/mL) to platelets was measured by flow cytometry after the addition of 20 μM of ADP. (A) Representative histograms. Fibrinogen bound to platelets treated with DMSO, myristic acid, and RGT at 250 μM (M + RGT), scrambled myr-GRT at 250 μM (myr-GRT), myr-RGT at 250 μM (myr-RGT), or with RGDS peptide (1 mM). The background fibrinogen binding was assessed using platelets treated without ADP (Control). (B) Statistical data were derived from quantitative results (means and SD) calculated from the ratios of mean fluorescence intensity (samples/control) of 3 separate experiments.
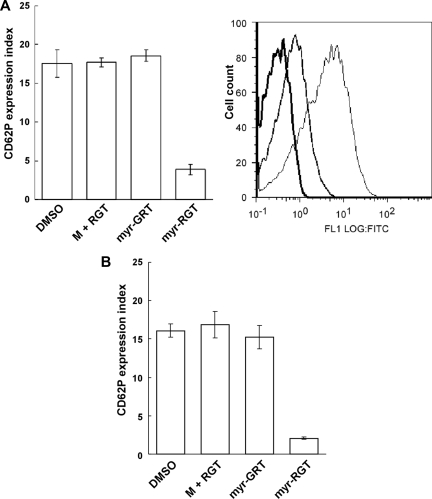
P-selectin expression on platelets treated with myr-RGT
The selective inhibition of the secondary wave of platelet aggregation by myr-RGT suggests that myr-RGT interferes with aggregation-dependent platelet granule secretion, an event known to require integrin outside-in signaling. To test this hypothesis, the effect of myr-RGT on P-selectin surface exposure as a marker of integrin-dependent α-granule release and adenosine triphosphate release as a marker of dense granule secretion was determined. Indeed, myr-RGT significantly inhibited P-selectin exposure on platelets (Figure 5A) or adenosine triphosphate release (not shown) induced by a low concentration of thrombin. An inhibition of approximately 80% of P-selectin expression compared with the control was observed at a peptide concentration of 250 μM. In contrast, no significant effect was observed with control peptides used at the highest concentration of 250 μM. Similarly, myr-RGT also inhibited ADP-induced P-selectin expression under stirring (Figure 5B). These results are in agreement with the inhibitory effects of myr-RGT on the secondary wave platelet aggregation, platelet spreading, and clot retraction, indicating that the functional outcome of integrin outside-in signaling was inhibited by myr-RGT.
Figure 5.
Effect of myr-RGT on platelet CD62P expression in the presence of agonists. The expression of CD62P on nontreated or peptide-treated platelets stimulated with agonists was analyzed by flow cytometry using an FITC-labeled monoclonal anti-CD62P antibody. (A) Data shown in the left pattern (mean ± SD) were derived from the ratio of the geometric mean fluorescence intensity measured for anti-CD62P antibody binding to thrombin-treated platelets, preincubated for 30 minutes with DMSO vehicle, nonmyristoylated RGT peptide (250 μM) plus myristic acid (M + RGT), scrambled myr-GRT (250 μM), or myr-RGT (250 μM), versus resting platelets (without thrombin treatment) and obtained from 3 separate experiments. Data shown in the right pattern are a representative figure of CD62P expression in the presence of thrombin on platelets preincubated with DMSO (fine line), myr-RGT (normal line), or on resting platelets (in the absence of thrombin; thick line). (B) Expression of CD62P on peptide-treated platelets stimulated with ADP under stirring conditions.
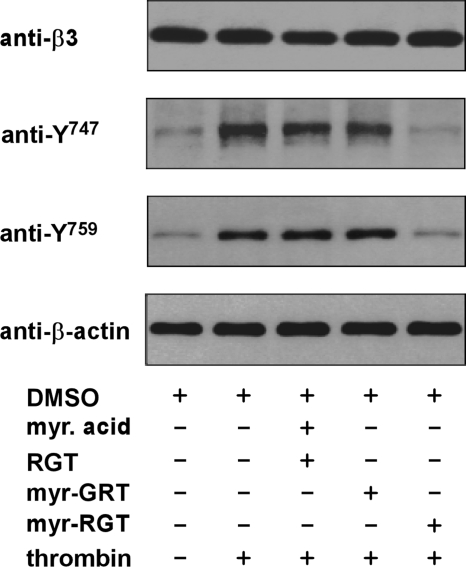
Regulation of integrin β3 cytoplasmic tyrosine phosphorylation by myr-RGT
Previous studies have well established that the postoccupancy events of integrin αIIbβ3 are closely related to the phosphorylation of 2 tyrosine residues present within the β3 cytoplasmic tail.6 Because Src interacts with the β3 cytoplasmic domain at the C-terminal RGT site and myr-RGT inhibited platelet function mediated by outside-in signaling, we hypothesized that the effect of myr-RGT might be associated with its inhibition of Src-mediated phosphorylation of β3 cytoplasmic domain. To test this hypothesis, we examined β3 tyrosine phosphorylation at Y747 and Y759 by Western blotting using antibodies specific for integrin β3 cytoplasmic sequences containing phosphotyrosine residues at position 747 or 759. As shown in Figure 6, unstimulated platelets exhibited a subtle level of tyrosine phosphorylation at residues 747 and 759, which presumably reflects weak platelet activation during platelet preparation. As expected, α-thrombin stimulation triggered the tyrosine phosphorylation of integrin β3 at Y747 and Y759. Addition of myr-RGT almost completely abolished phosphorylation of these tyrosines at a peptide concentration of 250 μM. In contrast, neither the scrambled myr-GRT peptide nor the nonmyristoylated RGT peptide plus myristic acid prevented tyrosine phosphorylation at these sites. These data clearly showed that myr-RGT attenuated the tyrosine phosphorylation of integrin β3 at Y747 and Y759, suggesting that myr-RGT inhibits the Src-dependent β3 phosphorylation, presumably by disrupting the interaction between β3 and Src or, otherwise, the consequent effects of the Src-β3 dissociation, such as the inhibitory effect on platelet aggregation.
Figure 6.
Effect of myr-RGT on thrombin-induced phosphorylation of integrin β3 cytoplasmic Y747 and Y759. Washed platelets were preincubated for 30 minutes with 250 μM of the different peptides as indicated. Thrombin (0.05 U/mL) was added to induce platelet aggregation with stirring at 1000 rpm for 1 minute. The platelets were then lysed in SDS-PAGE sample buffer and analyzed by Western blotting using monoclonal antibodies directed against the integrin β3 subunit extracellular domain (SZ21), β-actin, and polyclonal antibodies specific for the β3 integrin cytoplasmic sequences containing phosphorylated Y747 or Y759 residue, respectively.
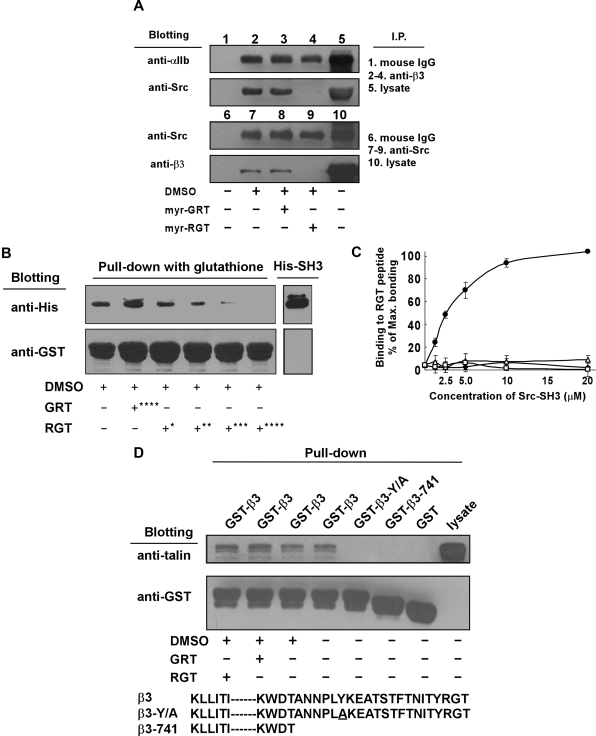
Effect of the RGT peptide on the interaction of platelet integrin β3 with SH3 domain of Src
To determine whether the RGT peptide interferes with Src interaction with β3, we performed coimmunoprecipitation experiments using platelet lysates in the presence of an excess of the RGT peptide. Figure 7A shows that Src as well as integrin αIIb, as expected, were coimmunoprecipitated with β3 by an anti-β3 antibody, but not by nonspecific control mouse IgG. Preincubation of the platelets with myr-RGT, but not the scrambled peptide, abolished coimmunoprecipitation of β3 with Src but had no effect on the coimmunoprecipitation of αIIb with β3. Similar results were obtained in experiments using an anti-Src antibody to precipitate Src-β3 complexes. Addition of RGT peptide to the Src-β3 complex after immunoprecipitation also disrupted Src-β3 association (not shown), indicating a direct effect of RGT peptide without relying on the platelet cytoplasmic environment. Further evidence for a direct effect of the RGT peptide on Src-β3 interaction was provided by pull-down experiments using purified recombinant GST-β3 and His-Src-SH3 proteins (Figure 7B) in which the RGT peptide dose-dependently inhibited Src-SH3 binding to the integrin β3 tail. These results led us to postulate that the binding of the RGT peptide directly induces dissociation of Src from integrin β3. As a proof of this hypothesis, Figure 7C depicts the direct binding of GST-Src-SH3 recombinant protein to immobilized RGT peptide. This binding was dose-dependent and almost reached saturation at a protein concentration of 20 μM. The specificity of the binding was evidenced by the negative reaction of GST-Src-SH3 with the scrambled GRT peptide. These data established that Src interaction with integrin β3 was abrogated by myr-RGT, which competes with β3 tail for binding Src. On the other hand, talin was positively detected in a GST-integrin β3 cytoplasmic domain pull-down experiment. Unlike what happened with the integrin β3-Src interaction, the RGT peptide could not affect integrin β3-talin interaction (Figure 7D). As controls, mutant integrin β3 cytoplasmic domains lacking the talin head binding site or bearing a point mutation, which is known to disrupt integrin β3-talin interaction, β3-741 and β3-Y/A, were unable to pull-down talin. These results indicate that the RGT peptide had no effect on talin binding to the integrin β3 cytoplasmic domain.
Figure 7.
Effect of myr-RGT on the interaction of integrin β3 cytoplasmic domain with Src or talin. (A) Platelets preincubated with 250 μM of myr-RGT or scrambled myr-GRT were lysed with lysis buffer, and the lysates of untreated or peptide-treated platelets were analyzed with an immunoprecipitation procedure as follows. The lysates were incubated with SZ21 antibody or nonspecific mouse IgG. After washing, the immune complexes were subjected to SDS-PAGE and probed by Western blotting using monoclonal antibodies SZ22 or 327 against integrin αIIb or c-Src, respectively (lanes 1-5). In another set of experiments, immunoprecipitation was performed with monoclonal antibody 327 and blotted with SZ21 or 327 (lanes 6-10). Representative results of 3 experiments are shown. (B) Glutathione-Sepharose 4B beads coated with GST-wild-type integrin β3 cytoplasmic tail fusion protein were incubated overnight with purified His-Src-SH3 in the presence of peptides as indicated. After wash, protein complexes were subjected to Western blot analysis with anti-His or anti-GST antibodies. Peptide concentrations: *62.5 μM; **125 μM; ***250 μM; ****500 μM. (C) Increasing concentrations of purified GST-Src-SH3 or GST protein were added to the microtiter wells coated with RGT or GRT peptide (20 μg/mL). Binding of the purified proteins to the peptides was detected by incubation with mouse anti-GST antibody, followed by horseradish peroxidase-conjugated antimouse Ig antibody. Specific binding was normalized by subtracting the OD (optical density) values of the blank wells from that of the sample wells. Results were presented as percentage of the maximal binding. Data were organized as binding of GST-Src-SH3 to RGT peptide (●), GST-Src-SH3 to GRT peptide (■), GST protein to RGT peptide (△), and GST protein to GRT peptide (□). (D) Glutathione-Sepharose 4B beads coated with GST-integrin β3 cytoplasmic tail fusion proteins were incubated overnight with platelet lysates in the presence of peptides as indicated at 4°C before being lysed by SDS sample buffer. Talin was detected with the monoclonal antibody 8d4. Anti-GST antibody binding was used to verify the loading of the β3 cytoplasmic tail fusion proteins. The increased electrophoretic mobility of GST-β3-741 documents the 21 residue truncation of this fusion protein.
Discussion
Previous observations made with the CHO cell model have demonstrated that αIIbβ3-dependent inside-out and outside-in signaling relies on distinct β3 cytoplasmic sequences.8,10,34–37 Using a cell-permeable peptide mimicking the β3 cytoplasmic C-terminal residues, the present study extends our understanding of the role of this β3 sequence in integrin signaling from CHO cell model to human platelets. Our major findings are as follows: (1) myr-RGT has an inhibitory effect on platelet functions regulated by outside-in signaling without affecting those by inside-out signaling; (2) the RGT peptide dissociates constitutively bound Src kinase from integrin β3 by directly binding to the SH3 domain of Src; and (3) the dissociation of Src kinase, but not talin, from integrin β3 results in inhibition of Src-related β3 cytoplasmic tyrosine phosphorylation and integrin outside-in signaling events. As the Y747 and Y759 phosphorylation is concomitant with platelet aggregation and the roles of Src in this process are multiple, the contribution of aggregation to the causal relationship between the myr-RGT-induced Src-β3 dissociation and the inhibition of integrin β3 tyrosine phosphorylation remains to be further elucidated in the future.
Different methods have been reported in the literature for peptide delivery into platelets. Platelet membrane becomes permeable to peptides by streptolysin O treatment38,39 or electroporation.40 In addition, chemical modifications, such as myristoylation or palmitoylation,21,23,41–46 or attachment of a hydrophobic sequence,24,47 promote the translocation of peptides across the platelet membrane. However, some of these methods have been shown to interfere with normal platelet functions; in particular, streptolysin O-treated platelets exhibited reduced adhesion.38,40 Here we have chosen the myristoylation approach because this peptide modification does not affect platelet adhesion. In platelet function assays, an inhibitory effect could be observed only in platelets treated with myr-RGT, whereas a scrambled myristoylated peptide or a nonmyristoylated RGT peptide was actually devoid of activity. In addition, myr-RGT did not cause platelet lysis as demonstrated by data with platelets in suspension or after adhesion. We accordingly conclude that the inhibitory effect of the RGT peptide on platelet function occurs intracellularly and is unlikely to be caused by a potential extracellular interaction with platelet surface receptors.
Among the integrin β3 cytoplasmic tail binding proteins, the cytoskeletal protein talin and the non-receptor tyrosine kinase Src are of particular interest, as the talin-integrin interaction is essential to the regulation of inside-out signaling3 and is also important for outside-in signaling,48 whereas Src is an important modulator of platelet functions mediated by outside-in signaling.49 The present work demonstrates that treatment of platelets with myr-RGT caused an inhibitory effect on typical integrin αIIbβ3 outside-in signaling events, including integrin-dependent platelet secretion, irreversible platelet aggregation, platelet stable adhesion, and spreading on immobilized fibrinogen as well as fibrin clot retraction mediated by washed platelets, whereas the inside-out signaling pathway was unaffected as evidenced by normal soluble fibrinogen binding and undisturbed first wave of platelet aggregation induced by low-dose agonists. These results suggested that the RGT peptide, which corresponds to the last 3 residues of the β3 cytoplasmic tail, was able to compete with the endogenous β3 integrin subunit for binding to cytoplasmic proteins involved in the outside-in signaling cascade, thereby exerting a dominant negative effect on this signaling pathway. As recently demonstrated by Arias-Salgado et al and others, the C-terminal RGT residues of β3 are required for Src binding to β3.8,9 However, these results did not allow to conclude whether the RGT sequence is sufficient for Src binding and whether adjacent sequences are also required. Our results provide evidence that an exogenous RGT peptide was able to cause a robust inhibition of the association between Src and β3 cytoplasmic tail (Figure 7A,B). The enzyme-linked immunosorbent assay data (Figure 7C) further showed that Src-SH3 directly bound to RGT peptide, suggesting that the regulatory effect of RGT peptide on outside-in signaling occurs through its direct binding to Src. These results clearly show that the β3 R760GT762 sequence is not only necessary but also sufficient for the β3 interaction with Src kinase. These data also provide important evidence that the C-terminal RGT sequence is critically important in integrin outside-in signaling in human platelets.
Based on our observation that the RGT peptide does not affect inside-out signaling (Figures 3B,4), it is intuitive for us that the RGT peptide should have no effect on talin head-β3 cytoplasmic tail interaction. We thus examined the interaction of β3 cytoplasmic domain model proteins with platelet talin by a pull-down procedure.8 Indeed, the talin-binding capacity of wild-type GST-β3 cytoplasmic tail fusion protein was not affected by the presence of the RGT peptide. (Figure 7D). These results support the conclusion that the R760GT762 sequence of the β3 tail is not involved in talin-β3 interaction and help to explain the inability of the RGT peptide to affect inside-out signaling. Importantly, our results provide a novel method to selectively interfere with integrin outside-in signaling in intact human platelets.
Integrin αIIbβ3 is an ideal antithrombotic target as its activation is the final common pathway mediating platelet aggregation in response to different agonists. However, existing integrin αIIbβ3 receptor antagonists that block ligand binding have to be used at a defined dose that has a significant inhibition of thrombosis without undue bleeding.50 In addition, some ligand-mimetic αIIbβ3 antagonists have been reported to cause conformational changes of αIIbβ3 and elicit outside-in signaling.51 The transgenic animals generated by targeting several key genes, including Gas6 receptors,28 PTP-1B,17 TSSC6,19 CD151,12 SLAM,13 or by introducing the DiYF mutation into integrin β3,6 all displayed a similar phenotype characterized by the absence of spontaneous bleeding and normal or only slightly prolonged tail bleeding time. These mice also exhibited the similar platelet dysfunction resulting from impaired outside-in signaling, and most of them showed a significantly reduced thrombosis potential in in vivo settings, such as photochemical denudation or collagen/epinephrine injection,28 ferric chloride,13,19 or laser injury.17 The RGT peptide described here caused a selective inhibition of outside-in signaling-related platelet functions similar to those exhibited by the platelets of the above-mentioned transgenic mice. This provides a rational basis for the design of novel antiplatelet agents with improved efficacy/side-effect (bleeding) profiles.
Acknowledgments
The authors thank Dr Zhu Chen and Dr Zhenyi Wang (Shanghai Institute of Hematology) for thoughtful advice and helpful suggestions throughout the study and for critical reading of this manuscript, Dr Hongli Wang (Shanghai Institute of Hematology) for helpful discussions, Dr Bob Lee (University of Illinois at Chicago) for peptide synthesis, and Ms Aleksandra Stojanovic (University of Illinois at Chicago) and Dr Xuetao Bai (Shanghai Institute of Hematology) for technical assistance.
This work was supported in part by grants from National Natural Science Foundation of China (30470741 and 30710103905, X.X.) and from Science and Technology Commission of Shanghai Municipality (06PJ14067, J.M.), and by grants (HL080264, HL062350, HL068819) from National Institutes of Health (X.D.). P.F. is the recipient of the American Heart Association Midwest Affiliate predoctoral fellowship.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.S. performed the experiments and prepared the manuscript; J.M. performed the experiments, analyzed data, and contributed to paper writing; J.Y. and P.F. performed the experiments and analyzed data; Y.L., H.L., and Z.R. performed the experiments; X.W. contributed to the organization of platelet aggregation experiments and data analysis; N.K. analyzed data and contributed to paper writing; S.C. contributed to concept discussion and to paper writing; X.D. and X.X. were responsible for conception, design, execution, and analysis of the studies and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaodong Xi, Shanghai Institute of Hematology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, 197 Second Ruijin Road, Shanghai 200025, China; e-mail: xixiaodong@shsmu.edu.cn; or Saijuan Chen, Shanghai Institute of Hematology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, 197 Second Ruijin Road, Shanghai 200025, China; e-mail: sjchen@stn.sh.cn; or Xiaoping Du, Department of Pharmacology, University of Illinois at Chicago, Chicago, IL 60612; e-mail: xdu@uic.edu.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin β tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 4.Vinogradova O, Velyvis A, Velyviene A, et al. A structural mechanism of integrin αIIbβ3 “in-side-out” activation as regulated by its cytoplasmic face. Cell. 2002;110:587–597. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 5.Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- 6.Law DA, DeGuzman FR, Heiser P, et al. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 7.Obergfell A, Eto K, Mocsai A, et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias-Salgado EG, Lizano S, Shattil SJ, Ginsberg MH. Specification of the direction of adhesive signaling by the integrin β cytoplasmic domain. J Biol Chem. 2005;280:29699–29707. doi: 10.1074/jbc.M503508200. [DOI] [PubMed] [Google Scholar]
- 9.Flevaris P, Stojanovic A, Gong H, et al. A molecular switch that controls cell spreading and retraction. J Cell Biol. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi X, Bodnar RJ, Li Z, Lam SC, Du X. Critical roles for the COOH-terminal NITY and RGT sequences of the integrin β3 cytoplasmic domain in inside-out and outside-in signaling. J Cell Biol. 2003;162:329–339. doi: 10.1083/jcb.200303120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xi X, Flevaris P, Stojanovic A, et al. Tyrosine phosphorylation of the integrin β3 subunit regulates β3 cleavage by calpain. J Biol Chem. 2006;281:29426–29430. doi: 10.1074/jbc.C600039200. [DOI] [PubMed] [Google Scholar]
- 12.Lau LM, Wee JL, Wright MD, et al. The tetraspanin superfamily member CD151 regulates outside-in integrin αIIbβ3 signaling and platelet function. Blood. 2004;104:2368–2375. doi: 10.1182/blood-2003-12-4430. [DOI] [PubMed] [Google Scholar]
- 13.Nanda N, Andre P, Bao M, et al. Platelet aggregation induces platelet aggregate stability via SLAM family receptor signaling. Blood. 2005;106:3028–3034. doi: 10.1182/blood-2005-01-0333. [DOI] [PubMed] [Google Scholar]
- 14.Pozgajova M, Sachs UJ, Hein L, Nieswandt B. Reduced thrombus stability in mice lacking the α2A-adrenergic receptor. Blood. 2006;108:510–514. doi: 10.1182/blood-2005-12-4835. [DOI] [PubMed] [Google Scholar]
- 15.Andre P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a β3 integrin-dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 16.Angelillo-Scherrer A, de Frutos PG, Aparicio C, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 17.Arias-Salgado EG, Haj F, Dubois C, et al. PTP-1B is an essential positive regulator of platelet integrin signaling. J Cell Biol. 2005;170:837–845. doi: 10.1083/jcb.200503125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maxwell MJ, Yuan Y, Anderson KE, et al. SHIP1 and Lyn kinase negatively regulate integrin αIIbβ3 signaling in platelets. J Biol Chem. 2004;279:32196–32204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]
- 19.Goschnick MW, Lau LM, Wee JL, et al. Impaired “outside-in” integrin αIIbβ3 signaling and thrombus stability in TSSC6-deficient mice. Blood. 2006;108:1911–1918. doi: 10.1182/blood-2006-02-004267. [DOI] [PubMed] [Google Scholar]
- 20.Wee JL, Jackson DE. The Ig-ITIM superfamily member PECAM-1 regulates the “outside-in” signaling properties of integrin αIIbβ3 in platelets. Blood. 2005;106:3816–3823. doi: 10.1182/blood-2005-03-0911. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboi S. Calcium integrin-binding protein activates platelet integrin αIIbβ3. J Biol Chem. 2002;277:1919–1923. doi: 10.1074/jbc.M110643200. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin αIIbβ3. J Biol Chem. 2001;276:42226–42232. doi: 10.1074/jbc.M106129200. [DOI] [PubMed] [Google Scholar]
- 23.Martin K, Meade G, Moran N, Shields DC, Kenny D. A palmitylated peptide derived from the glyco-protein Ibβ cytoplasmic tail inhibits platelet activation. J Thromb Haemost. 2003;1:2643–2652. doi: 10.1046/j.1538-7836.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu XY, Timmons S, Lin YZ, Hawiger J. Identification of a functionally important sequence in the cytoplasmic tail of integrin β3 by using cell-permeable peptide analogs. Proc Natl Acad Sci U S A. 1996;93:11819–11824. doi: 10.1073/pnas.93.21.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osdoit S, Rosa JP. Fibrin clot retraction by human platelets correlates with αIIbβ3 integrin-dependent protein tyrosine dephosphorylation. J Biol Chem. 2001;276:6703–6710. doi: 10.1074/jbc.M008945200. [DOI] [PubMed] [Google Scholar]
- 26.Podolnikova NP, Yakubenko VP, Volkov GL, Plow EF, Ugarova TP. Identification of a novel binding site for platelet integrins αIIbβ3 (GPIIbIIIa) and α5β1 in the gamma C-domain of fibrinogen. J Biol Chem. 2003;278:32251–32258. doi: 10.1074/jbc.M300410200. [DOI] [PubMed] [Google Scholar]
- 27.Merten M, Thiagarajan P. Role for sulfatides in platelet aggregation. Circulation. 2001;104:2955–2960. doi: 10.1161/hc4901.100383. [DOI] [PubMed] [Google Scholar]
- 28.Angelillo-Scherrer A, Burnier L, Flores N, et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J Clin Invest. 2005;115:237–246. doi: 10.1172/JCI22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prevost N, Woulfe DS, Tognolini M, et al. Signaling by ephrinB1 and Eph kinases in platelets promotes Rap1 activation, platelet adhesion, and aggregation via effector pathways that do not require phosphorylation of ephrinB1. Blood. 2004;103:1348–1355. doi: 10.1182/blood-2003-06-1781. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Zhang G, Marjanovic JA, Ruan C, Du X. A platelet secretion pathway mediated by cGMP-dependent protein kinase. J Biol Chem. 2004;279:42469–42475. doi: 10.1074/jbc.M401532200. [DOI] [PubMed] [Google Scholar]
- 31.Law DA, Nannizzi-Alaimo L, Phillips DR. Outside-in integrin signal transduction: αIIbβ3-(GP IIb IIIa) tyrosine phosphorylation induced by platelet aggregation. J Biol Chem. 1996;271:10811–10815. doi: 10.1074/jbc.271.18.10811. [DOI] [PubMed] [Google Scholar]
- 32.Arias-Salgado EG, Lizano S, Sarkar S, et al. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prevost N, Woulfe DS, Jiang H, et al. Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc Natl Acad Sci U S A. 2005;102:9820–9825. doi: 10.1073/pnas.0404065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes PE, O'Toole TE, Ylanne J, Shattil SJ, Ginsberg MH. The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J Biol Chem. 1995;270:12411–12417. doi: 10.1074/jbc.270.21.12411. [DOI] [PubMed] [Google Scholar]
- 35.Patil S, Jedsadayanmata A, Wencel-Drake JD, et al. Identification of a talin-binding site in the integrin β3 subunit distinct from the NPLY regulatory motif of post-ligand binding functions: the talin n-terminal head domain interacts with the membrane-proximal region of the β3 cytoplasmic tail. J Biol Chem. 1999;274:28575–28583. doi: 10.1074/jbc.274.40.28575. [DOI] [PubMed] [Google Scholar]
- 36.Ylanne J, Huuskonen J, O'Toole TE, et al. Mutation of the cytoplasmic domain of the integrin β3 subunit: differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J Biol Chem. 1995;270:9550–9557. doi: 10.1074/jbc.270.16.9550. [DOI] [PubMed] [Google Scholar]
- 37.Chen YP, O'Toole TE, Ylanne J, Rosa JP, Ginsberg MH. A point mutation in the integrin β3 cytoplasmic domain (S752→P) impairs bidirectional signaling through αIIbβ3 (platelet glycoprotein IIb-IIIa). Blood. 1994;84:1857–1865. [PubMed] [Google Scholar]
- 38.Hers I, Donath J, Litjens PE, van Willigen G, Akkerman JW. Inhibition of platelet integrin αIIbβ3 by peptides that interfere with protein kinases and the β3 tail. Arterioscler Thromb Vasc Biol. 2000;20:1651–1660. doi: 10.1161/01.atv.20.6.1651. [DOI] [PubMed] [Google Scholar]
- 39.Litjens PE, Gorter G, Ylänne J, Akkerman JW, van Willigen G. Involvement of the β3 E749ATSTFTN756 region in stabilizing integrin αIIbβ3-ligand interaction. J Thromb Haemost. 2003;1:2216–2224. doi: 10.1046/j.1538-7836.2003.00394.x. [DOI] [PubMed] [Google Scholar]
- 40.Litjens PE, Kroner CI, Akkerman JW, Van WG. Cytoplasmic regions of the β3 subunit of integrin αIIbβ3 involved in platelet adhesion on fibrinogen under flow conditions. J Thromb Haemost. 2003;1:2014–2021. doi: 10.1046/j.1538-7836.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- 41.Dai K, Bodnar R, Berndt MC, Du X. A critical role for 14–3-3ζ protein in regulating the VWF binding function of platelet glycoprotein Ib-IX and its therapeutic implications. Blood. 2005;106:1975–1981. doi: 10.1182/blood-2005-01-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin D, Murphy D, Reilly DF, et al. ICln, a novel integrin αIIbβ3-associated protein, functionally regulates platelet activation. J Biol Chem. 2004;279:27286–27293. doi: 10.1074/jbc.M402159200. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Jackson CW, Gruppo RA, Jennings LK, Gartner TK. The β3 subunit of the integrin αIIbβ3 regulates alphaIIb-mediated outside-in signaling. Blood. 2005;105:4345–4352. doi: 10.1182/blood-2004-07-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vinogradova O, Haas T, Plow EF, Qin J. A structural basis for integrin activation by the cytoplasmic tail of the αIIb-subunit. Proc Natl Acad Sci U S A. 2000;97:1450–1455. doi: 10.1073/pnas.040548197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephens G, O'Luanaigh N, Reilly D, et al. A sequence within the cytoplasmic tail of GpIIb independently activates platelet aggregation and thromboxane synthesis. J Biol Chem. 1998;273:20317–20322. doi: 10.1074/jbc.273.32.20317. [DOI] [PubMed] [Google Scholar]
- 46.Ginsberg M, Pierschbacher MD, Ruoslahti E, Marguerie G, Plow E. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985;260:3931–3936. [PubMed] [Google Scholar]
- 47.Wang Z, Leisner TM, Parise LV. Platelet α2β1 integrin activation: contribution of ligand internalization and the alpha2-cytoplasmic domain. Blood. 2003;102:1307–1315. doi: 10.1182/blood-2002-09-2753. [DOI] [PubMed] [Google Scholar]
- 48.Tremuth L, Kreis S, Melchior C, et al. A fluorescence cell biology approach to map the second integrin-binding site of talin to a 130-amino acid sequence within the rod domain. J Biol Chem. 2004;279:22258–22266. doi: 10.1074/jbc.M400947200. [DOI] [PubMed] [Google Scholar]
- 49.Shattil SJ. Integrins and Src: dynamic duo of adhesion signaling. Trends Cell Biol. 2005;15:399–403. doi: 10.1016/j.tcb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Phillips DR, Conley PB, Sinha U, Andre P. Therapeutic approaches in arterial thrombosis. J Thromb Haemost. 2005;3:1577–1589. doi: 10.1111/j.1538-7836.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- 51.Bassler N, Loeffler C, Mangin P, et al. A mechanistic model for paradoxical platelet activation by ligand-mimetic αIIbβ3 (GPIIb/IIIa) antagonists. Arterioscler Thromb Vasc Biol. 2007;27:e9–e15. doi: 10.1161/01.ATV.0000255307.65939.59. [DOI] [PubMed] [Google Scholar]