Abstract
Resolvin E1 (RvE1) is an omega-3 eicosapentaenoic acid (EPA)–derived lipid mediator generated during resolution of inflammation and in human vasculature via leukocyte-endothelial cell interactions. RvE1 possesses anti-inflammatory and proresolving actions. Here, we report that RvE1 in human whole blood rapidly regulates leukocyte expression of adhesion molecules. RvE1 in the 10- to 100-nM range stimulated L-selectin shedding, while reducing CD18 expression in both neutrophils and monocytes. When added to whole blood, RvE1 did not stimulate reactive oxygen species by either neutrophils or monocytes, nor did it directly stimulate cytokine/chemokine production in heparinized blood. Intravital microscopy (IVM) demonstrated that RvE1 rapidly reduced leukocyte rolling (∼ 40%) in venules of mice. In human platelet-rich plasma (PRP), RvE1 selectively blocked both ADP-stimulated and thromboxane receptor agonist U46619-stimulated platelet aggregation in a concentration-dependent manner. In contrast, Δ6,14-trans-RvE1 isomer was inactive. RvE1 did not block collagen-stimulated aggregation, and regulation of ADP-induced platelet aggregation was not further enhanced with aspirin treatment. These results indicate RvE1 is a potent modulator of leukocytes as well as selective platelet responses in blood and PRP, respectively. Moreover, the results demonstrate novel agonist-specific antiplatelet actions of RvE1 that are potent and may underlie some of the beneficial actions of EPA in humans.
Introduction
Inflammation is now widely recognized as a central component in several of the most prevalent human diseases in the developed world, including rheumatic diseases, asthma, diabetes, Alzheimer disease, and various cardiovascular conditions such as thrombosis, stroke, and atherosclerosis.1,2 Earlier results from humans and animals suggest that omega-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have diverse beneficial actions in many inflammatory disorders.3–5 The underlying cellular and molecular mechanisms responsible for their beneficial actions had remained for the most part elusive. Recently, we identified novel families of lipid mediators generated from EPA and DHA named resolvins and protectins, because they possess potent anti-inflammatory and proresolving actions. Collectively, these results emphasize the importance of ω-3 PUFAs as precursors for novel potent bioactive mediators.6–8 The name resolvin (resolution phase interaction products) was introduced for these endogenous bioactive compounds because they were originally identified in spontaneous self-limited murine inflammatory exudates in vivo.6,7
Resolvin E1 (RvE1) is also generated by human cell types during, for example, cell-cell interactions from EPA typified by endothelial cell and leukocyte interactions.6 In vascular endothelial cells, aspirin-acetylated COX-2 converts EPA to 18R-hydro(peroxy)-EPE, which is quickly reduced to 18R-HEPE. 18R-HEPE is then released from endothelium and rapidly transformed by activated human polymorphonuclear leukocyte (PMN) in the proximity to a bioactive trihydroxy-containing product, termed resolvin E1 (RvE1).6 In addition, RvE1 is also produced in vivo in humans via aspirin-independent routes via mechanisms involving P4506 and was recently found to be generated from EPA by Candida albicans.9 RvE1 biosynthesis in C albicans, for example, occurs in the absence of other cellular partners when EPA is present in the milieu and its biosynthesis is both aspirin and COX independent in these microbes. Thus, there are multiple biosynthetic pathways for RvE1 production in biologic systems, those being aspirin-triggered, P450-dependent, and/or de novo from microbial sources. At the cellular level, resolvin E1 (RvE1) reduces PMN infiltration in vivo and blocks transmigration of human PMNs in vitro.6 These responses evoked by RvE1 are mechanisms of interest in the resolution of inflammation. RvE1 also stimulates clearance of microbes in vivo at the tissue level.9,10 Total organic synthesis confirmed the original structural assignment and bioactions as well as established the complete stereochemistry of natural RvE1, 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid.8
RvE1 possesses unique counterregulatory actions, including reducing PMN transendothelial migration, dendritic cell migration, and IL-12 production in vitro, as well as potent protective actions in vivo reducing inflammation and tissue damage in murine peritonitis, dermal inflammation, and colitis.8,11 In a rabbit model of periodontal disease, RvE1 reduces periodontal inflammation and protects from inflammation-induced bone loss.12 Earlier findings from humans in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI) study demonstrated reduced mortality from cardiovascular complications (eg, cardiac death, coronary death) in more than 11 000 cardiovascular patients taking ω-3 PUFAs.3,4 It is noteworthy that, given the cardiovascular status of these patients, all were taking aspirin.3,4 Because RvE1 is present in human plasma,8 RvE1 actions with blood cells are of interest, and thus we systematically assessed the actions of RvE1 in whole blood. Here, we report that RvE1 regulates cell adhesion molecules on both PMNs and monocytes in whole blood, reduces leukocyte rolling in vivo, and blocks platelet aggregation to certain agonists.
Methods
Antibodies and reagents
Fluorescein isothiocyanate (FITC)–conjugated mouse anti–human CD62L (BD Biosciences, Rockville, MD), R-phycoerythrin (R-PE)–conjugated mouse anti–human CD18 (Cell Sciences, Canton, MA), and RPE-Cy5–conjugated mouse anti–human CD14 (Gene Tex, San Antonio, TX) were purchased; appropriately labeled, class-matched mouse IgGs were used as negative controls for CD18 and CD62L (BD Biosciences) staining. ChemR23 antibody was from R&D Systems (Minneapolis, MN). Red blood cell (RBC) lysis buffer was purchased from eBioscience (San Diego, CA) and carboxy-2,7-dichlorodihydrofluorescein diacetate (carboxy-H2DCF-DA), from Invitrogen (Carlsbad, CA). ADP (Sigma-Aldrich, St Louis, MO), collagen (Chronolog, Havertown, PA), and U46619 (Cayman Chemical, Ann Arbor, MI) were used as agonists for platelet aggregation experiments. RvE1 and Δ6,14-trans-RvE1-free acids were prepared as in Arita et al8 from their corresponding carboxy methyl esters; each was prepared by total organic synthesis (Prof Nicos Petasis, University of Southern California [Los Angeles], Leader, Organic Synthesis Core of the Specialized Center for Research P50-DE016191).
Whole blood
Fresh venous blood (anticoagulated with 10 U/mL sodium heparin for flow cytometric experiments or with sodium citrate in a 1:10 ratio for platelet aggregation experiments) was collected from healthy nonsmoking volunteers who denied taking any drugs for at least 2 weeks before the experiments. For some experiments, healthy volunteers had declared taking low-dose aspirin. Informed consent was obtained from each volunteer in accordance with the Declaration of Helsinki. The protocol was approved by the Brigham and Women's Hospital IRB (protocol number 88-02642, approved December 5, 2006). Coded deidentified blood from healthy volunteers for these experiments was also obtained from Children's Hospital Boston (Boston, MA).
Cell surface antigen expression
Whole blood aliquots were incubated with either increasing concentrations of RvE1 for 30 minutes or with 30 nM RvE1 for different time periods in a 37°C water bath with gentle mixing every 5 minutes. Alternatively, whole blood was incubated with the proper vehicle control for RvE1 following the same experimental conditions as for RvE1 incubation. Red blood cells were lysed using 1× RBC lysis buffer at a 25:1 ratio with blood for 10 minutes on ice. Direct immunofluorescence labeling was performed next using mixtures of 3 antibodies (anti–human CD62L, CD18, or CD14 in combination with the corresponding isotype controls) in a 1:100 ratio in fluorescence-activated cell sorting (FACS) buffer for 30 minutes on ice. After washing, cell pellets were resuspended in FACS buffer and analyzed using FACSort (Becton Dickinson, Franklin Lakes, NJ) and Cellquest software (Becton Dickinson). Monocytes and neutrophils were gated using forward scatter and anti-CD14 staining.
Reactive oxygen species and plasma cytokines/chemokines
Intracellular reactive oxygen species (ROS) were monitored as in Bass et al.13 Briefly, whole blood was initially incubated with 50 μM carboxy-H2DCF-DA for 15 minutes at 37°C with gentle agitation. Whole blood was incubated with increasing concentrations (10-1000 nM) of either RvE1 or fMLP for 15 minutes. Next, direct immunofluorescence labeling with anti-CD14 antibody for 30 minutes on ice was performed to differentiate between PMNs and monocytes. To assess potential changes in cytokines or chemokines, human whole blood anticoagulated with heparin was incubated with RvE1 (100 nM) or vehicle in a water bath at 37°C. These incubations were stopped at 4 hours by placing on ice and plasma was collected (centrifugation at 350g, 15 minutes). Aliquots of the collected plasma were taken for specific chemokine and cytokine analyses as in Bannenberg et al14 using a custom-designed SearchLight Proteome Array (Pierce Boston Technology Center, Woburn, MA).
Analysis of leukocyte behavior by intravital microscopy
The right jugular vein of anesthetized mice was catheterized with polyethylene tubing, and the right cremaster muscle was surgically prepared and superfused with sterile Ringer injection solution as described previously.15 Preparations were transferred to an intravital microscope (Mikron Instruments, San Marcos, CA), and 10 mL/kg saline containing 0.2% rhodamine 6G (Molecular Probes) was injected intravenously. Using a Rapp OptoElectronic SP-20 xenon flash lamp system (Hamburg, Germany) and QImaging Rolera-MGi EMCCD camera (Surrey, BC) on an intravital microscope, 1-minute recordings of fluorescent leukocytes in venular trees consisting of 2 to 4 branches were made (control period). After a 15-minute control period, RvE1 was injected intravenously as a bolus (100 ng/mouse). The same venular trees were recorded and the leukocyte rolling fraction was measured offline by determining the percentage of interacting leukocytes in the total flux of passing leukocytes in each venule.
Platelet-rich plasma and platelet aggregation
Platelet-rich plasma was prepared as in Zhou and Schmaier.16 Briefly, human whole blood was collected in sodium citrate and PRP obtained after centrifugation (110g, 15 minutes). Supernatants were removed and placed into polypropylene tubes (Falcon; Becton Dickinson). PRP was adjusted to 200 to 400 × 109 platelets/L (200 to 400 × 103) platelets/μL. Platelet-poor plasma (PPP) was prepared by centrifuging anticoagulated blood (1880g, room temperature, 20 minutes). PPP counts were adjusted similarly to the PRP. Platelet aggregation was completed within 3 hours after venipuncture. Isolated PRP was first incubated (15 minutes, 37°C) with RvE1, Δ6,14-trans-RvE1, or vehicle before addition of platelet agonists: 10 μM ADP, 1.5 μg/mL collagen, or 0.5 μM U46619. Platelet aggregation was monitored by changes in light transmittance of stirred (400 rpm) PRP after additions using an aggregometer (dual-channel aggregometer; Chronolog).
Recombinant thromboxane receptor radioligand binding
[3H]-SQ-29548 binding was carried out with HEK293 cells17 transiently transfected with recombinant thromboxane receptor TPα using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA). Twenty-four hours later, cells were harvested and suspended in Dulbecco phosphate-buffered saline with CaCl2 and MgCl2 (DPBS++). Aliquots (∼ 0.2 × 106 HEK293) were incubated with 4 nM [3H]-SQ-29548 (∼ 20 000 cpm, specific activity ∼ 43.6 Ci (1.6 × 1012 Bq)/mmol; Perkin Elmer, Boston, MA) in the absence or presence of increasing concentrations of unlabeled homoligand (SQ-29548) or heteroligand (RvE1) for 60 minutes at 4°C. The bound and unbound radioligands were separated by filtration through Whatman GF/C glass microfiber filters (Fisher, Pittsburgh, PA). Filters were washed twice with 5 mL ice-cold washing buffer (10 mM Tris/HCl, pH 7.5). The radioactivity retained on the filter was determined using a scintillation counter (Beckman Coulter, Fullerton, CA). Nonspecific binding was determined in the presence of 1000 nM unlabeled homoligand.
Data analysis
The significance of difference between groups was evaluated using a 2-tailed Student t test. A P value less than .05 was considered statistically significant.
Results
RvE1 modulates adhesion molecules on PMNs and monocytes in human whole blood
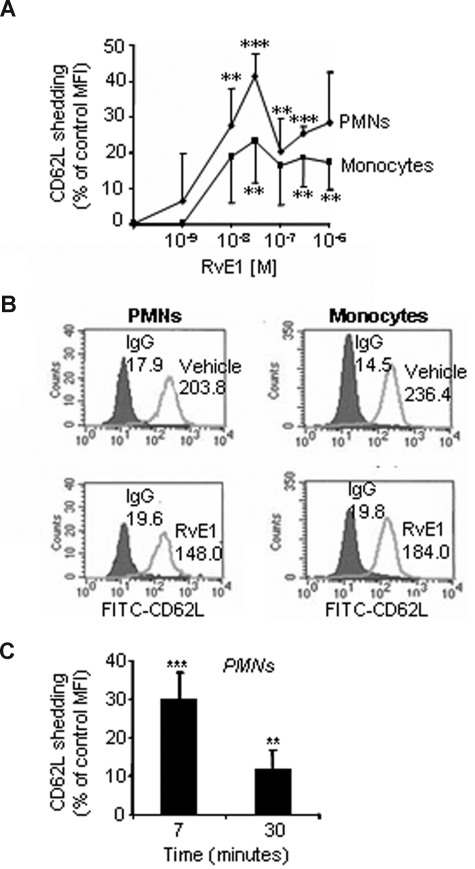
Because RvE1 is endogenously generated in whole blood and inflammatory exudates,6,8 we first determined whether RvE1 acts in human whole blood. Leukocyte recruitment to inflamed areas requires a precise sequence of events that initially involves the interaction of leukocytes with activated endothelial cells via regulated expression of surface adhesion molecules.18 The contact with inflamed endothelium facilitates rolling and temporary arrest of circulating leukocytes on the endothelium surface via L-selectin (CD62L), a step that is essential for leukocyte recruitment.19,20 RvE1 was added to heparinized whole blood freshly collected from healthy human volunteers. RvE1 stimulated L-selectin shedding, compared with vehicle, in a concentration-dependent manner in whole blood that reached maximum levels at approximately 30 nM (Figure 1A). Representative histograms demonstrate the impact of RvE1 on CD62L expression in both PMNs and monocytes (Figure 1B). RvE1 (30 nM) stimulated a rapid L-selectin shedding from peripheral blood PMNs evident within minutes, giving 30.3% plus or minus 6.7% reduction in CD62L expression compared with vehicle alone added to whole blood (Figure 1C). Monocytes displayed a similar initial L-selectin shedding in response to RvE1 (9.2% ± 10.0%, at 7 minutes) compared with vehicle alone (data not shown). These results indicate that RvE1 acts directly on both monocytes and PMNs in human whole blood.
Figure 1.
RvE1 regulates L-selectin shedding on PMNs and monocytes in whole blood. Heparinized human whole blood was incubated (30 minutes, 37°C) with increasing concentrations of RvE1 or vehicle alone, then stained with anti-CD62L (or L-selectin) for flow cytometry. CD14 was used to identify monocyte populations and PMNs were identified based on cellular morphology. (A) RvE1 stimulates L-selectin shedding in PMNs ( ) and monocytes (■): concentration dependence. (B) Representative histograms of CD62L expression on PMNs and monocytes in whole blood after 7-minute incubation with RvE1 (30 nM). Numbers on plots are the mean fluorescence intensity of CD62L-positive PMNs or monocytes. (C) Percentage CD62L shedding from human PMNs compared with vehicle alone. Results are expressed as a percentage shedding of control mean fluorescence intensity (MFI) with the means plus or minus SEM from 3 to 6 healthy donors. **P < .005; ***P < .001.
) and monocytes (■): concentration dependence. (B) Representative histograms of CD62L expression on PMNs and monocytes in whole blood after 7-minute incubation with RvE1 (30 nM). Numbers on plots are the mean fluorescence intensity of CD62L-positive PMNs or monocytes. (C) Percentage CD62L shedding from human PMNs compared with vehicle alone. Results are expressed as a percentage shedding of control mean fluorescence intensity (MFI) with the means plus or minus SEM from 3 to 6 healthy donors. **P < .005; ***P < .001.
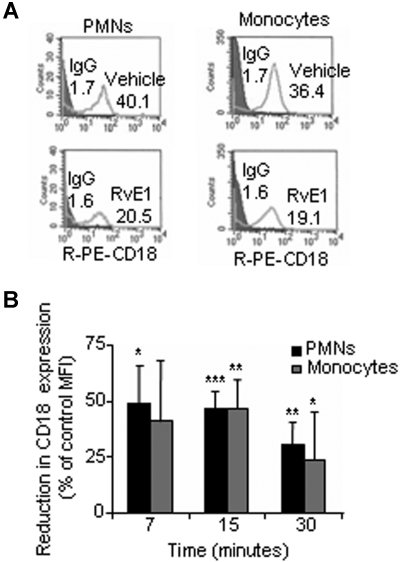
Rapid L-selectin shedding is usually associated with early leukocyte activation and rolling, whereas the CD18 integrins (Mac-1 and LFA-1)2,18 are involved in the subsequent stable adhesion and transmigration of neutrophils via binding to the endothelial counterreceptors, the intercellular adhesion molecule 1 and 2.19 To determine whether RvE1 has a role in this step, we next investigated whether RvE1 modulates CD18 expression. The representative histograms shown in Figure 2A demonstrate that RvE1 reduces CD18 expression on both PMNs and monocytes. Addition of RvE1 to whole blood significantly reduced CD18 expression on PMNs, giving 49.3% plus or minus 16.7%, 47.1% plus or minus 7.3%, and 30.7% plus or minus 9.6% reduction in CD18 expression after 7, 15, and 30 minutes, respectively, compared with whole blood incubated with vehicle alone (Figure 2B). RvE1 also reduced CD18 surface expression on monocytes, which was maximal at 15 minutes, giving 46.5% plus or minus 12.8% reduction, compared with cells from vehicle treatment (Figure 2B).
Figure 2.
RvE1 reduces CD18 expression on PMNs and monocytes in whole blood. Human whole blood was incubated (30 minutes, 37°C) with RvE1 (30 nM) or vehicle alone then stained with anti-CD18 for flow cytometry. (A) Representative fluorescence histograms of CD18 expression on PMNs and monocytes with RvE1. Numbers on plots are the mean fluorescence intensity of CD18-positive PMNs or monocytes. (B) RvE1 reduces CD18 surface expression on human PMNs (■) and monocytes ( ) compared with vehicle. Results are expressed as percentage reduction in control MFI (means ± SEM from 3 to 6 healthy donors). *P < .05; **P < .005; ***P < .001.
) compared with vehicle. Results are expressed as percentage reduction in control MFI (means ± SEM from 3 to 6 healthy donors). *P < .05; **P < .005; ***P < .001.
RvE1 reduces leukocyte rolling
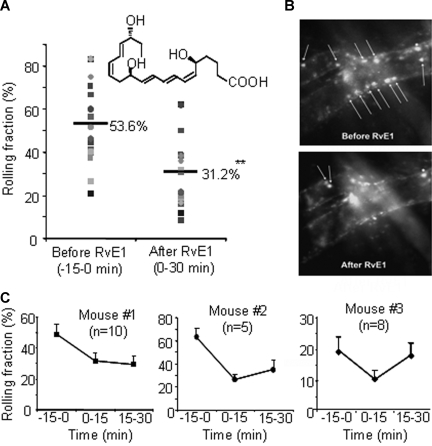
The actions of RvE1 on leukocyte rolling in vivo were investigated using intravital microscopy of mouse cremaster muscle (Figure 3).15 The leukocytes displayed slow rolling and characteristic adherence to the venules. Administration of RvE1 gave approximately 40% reduction in leukocyte rolling (Videos S1,S2, available on the Blood website; see the Supplemental Materials link at the top of the online article), indicating that RvE1 regulates leukocyte-endothelial interactions in vivo.
Figure 3.
Resolvin E1 rapidly reduces leukocyte rolling in mouse cremaster muscle venules. (A) The rolling fraction (percentage of rolling cells in the total flux of passing leukocytes in each venule) in 10 venules from mouse no. 1 (■) and 5 venules from mouse no. 2 (●). The horizontal lines are the means of rolling fractions from 15 venules of mice 1 and 2 before (−15 to 0 minutes) and after (0-30 minutes) RvE1 injection. The shaded shapes indicate each venule from the mice. (B) Representative recordings of fluorescent leukocytes in venular trees before and after RvE1 injection. Arrows show rolling leukocytes. (C) The rolling fractions (means ± SEM) from 3 mice. N indicates the number of venules analyzed in each mouse. **P < .005.
Reactive oxygen species production and cytokines/chemokines
Leukocytes generate ROSs in response to stimuli.21 Next, we tested whether RvE1 stimulates intracellular generation of ROSs in situ. Human whole blood was incubated with the ROS indicator carboxy-H2DCF-DA for 15 minutes followed by addition of increasing concentrations (10-1000 nM) of either RvE1 or fMLP. Carboxy-H2DCF-DA is a cell-permeable indicator that is trapped within cells by esterase cleavage, and thus susceptible to oxidation by ROSs and monitored by flow cytometry. RvE1 alone did not increase ROS production within either neutrophils or monocytes in the concentration range tested in whole blood (Figure S7). For direct comparison, formyl-Met-Leu-Phe (fMLP) displayed aconcentration-dependent increase in intracellular ROS production in both PMNs and monocytes.
Because cytokines and chemokines are important mediators involved in both the initiation and prolongation of inflammation as well as the resolution phase,2 we monitored an array of cytokines and chemokines in whole blood to assess whether RvE1 regulates their plasma levels. Freshly isolated human whole blood collected in heparin from healthy volunteers was incubated with either RvE1 (100 nM) or vehicle alone (4 hours at 37°C), and plasma cytokine/chemokine levels were determined. RvE1 alone did not stimulate the appearance of chemokines or cytokines (ie, IL-10, IFN, IL-1β, IL-12, IL-13, MCP-1, MIP-1α, GroA) in these incubations. In whole blood, RvE1 did give reduced levels of TNFα and IL-8 (Table S1). Together, these results demonstrate that RvE1 does not stimulate proinflammatory mediators (eg, ROSs) or proinflammatory chemokines (eg, IL-8, TNF-α, MCP-1, RANTES, GroA); yet in this concentration range, RvE1 regulates leukocyte adhesion in vitro and in vivo, suggesting that RvE1 is an agonist of nonphlogistic activation of leukocytes.
RvE1 selectively blocks ADP-induced platelet aggregation
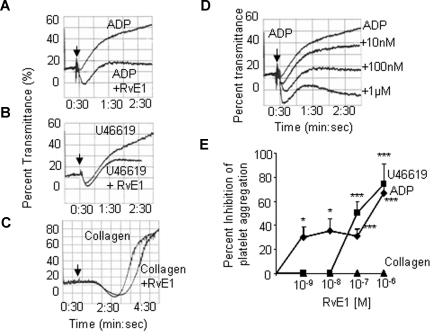
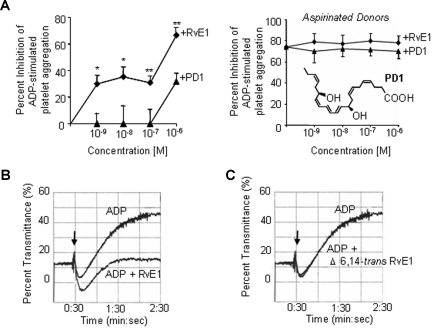
Platelets are present when inflammation develops and play an important role at several stages during inflammation, partly by close interaction with leukocytes and their released factors.22 Upon activation, platelets undergo rapid shape change and aggregate. Next, we isolated platelet-rich plasma and tested RvE1 (Figure 4) and an equiconcentration of its precursor EPA (data not shown) for their impact on platelet aggregation. Neither EPA nor RvE1 alone initiated platelet aggregation. Of interest, RvE1 blocked ADP-stimulated platelet aggregation in a concentration-dependent manner as shown in Figure 4A,D.
Figure 4.
RvE1 is a potent inhibitor of human platelet aggregation in PRP. PRP collected from healthy donors was incubated with RvE1 (15 minutes, 37°C).  denotes addition of ADP (10 μM; A), U46619 (0.5 μM; B), or collagen (1.5 μg/mL, C). Platelet aggregation was determined using an aggregometer. (D) RvE1 blocks ADP-induced platelet aggregation in a concentrationdependent fashion (representative of n = 6 donors). (E) Percentage inhibition of agonist-induced platelet aggregation by RvE1. ♦ indicates ADP (10 μM) plus RvE1 (n = 6 donors); ■, U46619 (0.5 μM) plus RvE1 (n = 6); and ▲, collagen (1.5 μg/mL) plus RvE1 (n = 4). Results are means plus or minus SEM. *P < .05; ***P < .001.
denotes addition of ADP (10 μM; A), U46619 (0.5 μM; B), or collagen (1.5 μg/mL, C). Platelet aggregation was determined using an aggregometer. (D) RvE1 blocks ADP-induced platelet aggregation in a concentrationdependent fashion (representative of n = 6 donors). (E) Percentage inhibition of agonist-induced platelet aggregation by RvE1. ♦ indicates ADP (10 μM) plus RvE1 (n = 6 donors); ■, U46619 (0.5 μM) plus RvE1 (n = 6); and ▲, collagen (1.5 μg/mL) plus RvE1 (n = 4). Results are means plus or minus SEM. *P < .05; ***P < .001.
In addition to ADP, RvE1 also stopped platelet aggregation stimulated by U46619 (0.5 μM), a thromboxane receptor (TP) agonist, with similar kinetics (Figure 4B,E). In contrast, RvE1 did not affect (1.5 μg/mL) collagen-stimulated platelet aggregation, indicating an agonist-selective action of RvE1 on platelet aggregation (Figure 4C,E). RvE1 (1.0 nM-1.0 μM) also blocked ADP-stimulated thromboxane release from human PRP (n = 4; data not shown). In parallel, we also assessed protectin D1 (PD1), a novel DHA-derived mediator.7,23 Unlike platelet responses with RvE1, PD1 at 1.0-nM to 100-nM concentration range had no direct action on platelet aggregation stimulated with all 3 agonists (ADP, U46619, and collagen) independently (n = 3; Figure 5A left panel). Next, we determined whether aspirin therapy had an impact on RvE1's antiplatelet actions. As expected, low-dose aspirin inhibited ADP-stimulated platelet aggregation, and RvE1 did not enhance aspirin inhibitory action (Figure 5A right panel).
Figure 5.
RvE1 stereoselectively blocks ADP-stimulated platelet aggregation in PRP. (A left) Percentage inhibition ADP (10 μM)–induced platelet aggregation. (A right) PRP from aspirinated healthy donors was incubated with RvE1 or protectin D1, PD1 (15 minutes, 37°C); see inset. (B,C) Direct comparison of RvE1 (100 nM) and Δ6,14-trans-RvE1 (100 nM). Results are percentage inhibition of ADP-stimulated PRP; means plus or minus SEM from 3 to 6 experiments with different PRP preparations. *P < .05; ***P < .001.
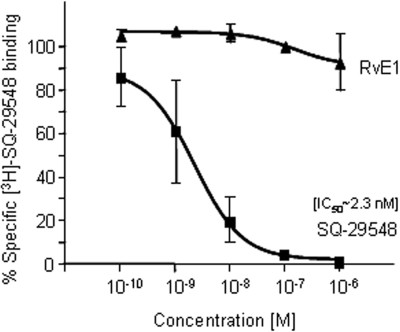
To address whether RvE1's actions on platelets were stereoselective, RvE1 was directly compared with its biologically inactive isomer, the Δ6,14-trans-RvE1 isomer8 in platelet aggregation. In Figure 5B, RvE1 markedly reduced ADP-stimulated platelet aggregation, whereas its isomer Δ6,14-trans-RvE1 at 100 nM did not (Figure 5C). Taken together, these results demonstrate a selective action for RvE1 in attenuating human platelet aggregation in PRP. Because RvE1 blocked TP receptor agonist U46619-stimulated platelet aggregation, we examined whether RvE1 directly binds to TPα receptor. Using a radiolabeled TP receptor antagonist SQ-29548, we demonstrated that RvE1 (0.1 nM-1 μM) did not compete with SQ-29548 for TPα-specific binding (Figure 6). Thus, these results rule out the possibility that RvE1 acts at the TPα receptor as a competitive ligand to block platelet activation.
Figure 6.
RvE1 does not directly compete with thromboxane receptor ligand: [3H]-SQ-29548–specific binding with recombinant TPα. HEK293 cells were transiently transfected with TPα. Twenty-four hours later, cells were collected, and [3H]-SQ-29548 binding was carried out. Transfected HEK293 cells (∼ 0.2 × 106) were incubated with [3H]-SQ-29548 (∼ 4.5 nM) for 60 minutes at 4°C in the presence or absence of unlabeled SQ-29548 or RvE1-free acid (0.1 to 1000 nM). Bound and unbound radioligands were separated by filtration and specific binding was determined. Results are means plus or minus SEM from duplicates of n = 3.
Discussion
Dietary supplementation with omega-3 fatty acids (ie, EPA and DHA) and treatment with aspirin reduces mortality in patients after myocardial infarction as well as improves the outcome of several chronic inflammatory and cardiovascular diseases.4,24,25 In the present report, we demonstrate new actions of RvE1 in whole blood, namely, RvE1 (1) regulates cell surface adhesion molecules that are essential for leukocyte extravasation, (2) reduces leukocyte rolling in venules, and (3) reduces both ADP and thromboxane receptor-stimulated platelet aggregation. These new RvE1 actions are at regulatory points and hallmarks that can each contribute to the resolution of inflammation, thrombosis, and vascular pathophysiology.
In whole blood, at concentrations as low as 30 nM, RvE1 stimulated L-selectin shedding that was evident within minutes, concomitant with prolonged reduction of CD18 expression on both human PMNs and monocytes. L-selectin is implicated in the initial steps during leukocyte recruitment to the inflammatory sites, that is, tethering and rolling of leukocytes on the endothelium before firm adhesion and transmigration.20 L-selectin shedding during rolling interactions is essential for limiting leukocyte aggregation and accumulation at sites of inflammation.26 In addition, L-selectin shedding from circulating PMNs in vivo reduces ongoing inflammatory response by preventing PMN accumulation.20 Thus, RvE1-induced L-selectin shedding together with the reduction in CD18 surface expression (Figures 1,2) on human PMNs and monocytes as well as the reduced rolling in murine venules in vivo (Figure 3) provide cellular mechanism(s) underlying in vivo findings demonstrating that RvE1 attenuates neutrophil recruitment to the inflammatory sites in murine models of acute inflammation including peritonitis, colitis, and the dermal air pouch.6,8,11 Together, these results also offer molecular mechanisms that may explain earlier clinical findings with EPA supplementation. For example, Sperling et al reported that dietary ω-3 PUFA supplementation inhibits human PMN chemotaxis,27 and Bang et al showed antithrombotic effects of PUFA, particularly with EPA.28
It is noteworthy that whole blood from healthy volunteers incubated with RvE1 alone did not stimulate changes in the levels of most of the major human cytokines/chemokines (ie, IL-1β, IL-12, IL-13, MIP-1α, GroA, etc; Table S1). In contrast, RvE1 did consistently reduce the levels of both IL-8 and TNFα production. These 4-hour incubation conditions were shown earlier to be an appropriate duration to assess plasma cytokine/chemokine release in whole blood.29,30 RvE1 at sites of inflammation in vivo negatively regulates most proinflammatory cytokines and chemokines,10,14 as it does also with human macrophages in vitro.31 At 100 nM, RvE1 gives a trend in whole blood toward increased IL-10 and IFN-gamma in amounts that may not prove to be physiologically relevant (Table S1). RvE1 blocks superoxide anion release from isolated PMNs obtained from both periodontal disease patients and healthy volunteers.12 On its own, RvE1 does not stimulate intracellular ROSs in human PMNs. The present results demonstrating that RvE1 does not stimulate ROSs in whole blood from either PMNs or monocytes are consistent with earlier findings with isolated PMNs.9,12 Of interest, RvE1 enhances the production of intraphagosomal vacuole ROSs generated with phagocytosis of the yeast C albicans.9 Thus, taken together these results provide evidence for RvE1 as a nonphlogistic agonist in human whole blood from healthy volunteers and are consistent with RvE1's anti-inflammation and proresolving actions in murine systems.8
Platelets are critical in coagulation, atherogenesis, and wound healing as well as in inflammation. Platelet-platelet interactions are essential in thrombosis, which has further consequences on platelet-leukocyte and platelet-endothelium interactions.22 These active cell-cell communications are responsible for and provide the links between thrombosis, inflammation, and atherogenesis.32,33 In view of the beneficial impact of EPA and aspirin in the cardiovascular arena, it was of particular interest to investigate whether RvE1 has a direct impact on platelets. The present results clearly demonstrate that RvE1 displays both a stereoselective and concentration-dependent antiplatelet aggregation action with specific platelet agonists. RvE1 blocks both ADP- and U46619-stimulated platelet aggregation in human PRP. In this context, earlier studies concluded that high dose with prolonged dietary EPA supplementation inhibits platelet aggregation, which was attributed to alterations in the pattern of thromboxane and prostacyclin biosynthesis.34,35 Of interest, exogenous EPA (125 μg) inhibits ex vivo platelet aggregation in response to ADP.34 We also found that RvE1 (300 nM) alone diminishes P-selectin expression (∼ 35% reduction, n = 3 separate donors) in human whole blood cells compared with vehicle-treated whole blood. This finding implies that RvE1 might also block initial platelet-leukocyte interactions that are bridged via P-selectin as well as the firm adhesion step operated by β2 integrin (CD18), thereby potentially also regulating platelet-facilitated leukocyte transendothelial migration.32 Low-dose aspirin is a well-recognized antithrombotic drug with known inhibitory actions on platelet aggregation through the inhibition of platelet TXA2 production.22,36 We found that RvE1 displays inhibitory actions on platelet aggregation similar to those of aspirin, yet RvE1 added to aspirinated PRP did not afford additional reduction of platelet aggregation compared with aspirin alone. It is noteworthy that the amounts of RvE1 used in the present experiments in vitro appear to be within the physiologic range produced in healthy humans.8 For example, in healthy individuals taking 160 mg aspirin together with EPA (1 g) and DHA (0.7 g), the plasma values ranged from 0.1 to 0.4 ng RvE1/mL,8 which corresponds to a nanomolar concentration range that is commensurate with the RvE1 actions identified in the present study in human whole blood and human PRP.
Along these lines, a randomized double-blind clinical trial with 128 healthy subjects allocated to placebo or to 81-mg, 325-mg, or 650-mg daily doses of aspirin for 8 weeks showed that the plasma levels of 15-epi-lipoxin A4 were significantly increased in the 81-mg aspirin group, with borderline increases in the 325-mg and no apparent additional significant increases in the 650-mg group.37 These results indicate that low-dose aspirin is sufficient and promotes the production of proresolving mediators such as 15-epi-LXA4 and, by comparison, aspirin-triggered levels of RvE1, likely via acetylated COX-2 in vivo in healthy individuals.
A portion of RvE1 is also produced in humans via routes that are neither COX-2 nor aspirin dependent8 and likely involve P450 production of the 18R-HEPE precursor to RvE1. Nonmammalian cells can produce RvE1 in that C albicans biosynthesizes resolvins from exogenous precursors that are identical to those produced by human cells. RvE1 biosynthesis in C albicans occurs in the absence of other cellular partners and is thus aspirin and COX independent.9 Thus, microbes can also contribute to the production of RvE1 in humans.
Lipoxin A4 (LXA4), an anti-inflammatory and proresolving mediator generated from arachidonic acid, also displays potent regulatory action in human whole blood by modulating both L-selectin and CD11/18 expression on resting leukocytes.38 The aspirin-triggered lipoxin A4 analog, 15-epi-16-p-fluorophenoxy-LXA4, decreases both PMN-platelet and PMN-PMN adhesion, which are interactions dependent on P-selectin, L-selectin, and CD18,39 in human whole blood at 14 minutes after shear. In this context, RvE1 and LXA4 both exhibit anti-inflammatory and proresolving properties in vivo, yet each acts at different receptors and checkpoints, thereby regulating selective molecular and cellular components to promote catabasis (ie, return to homeostasis).14 In the case of RvE1, ChemR23 and leukotriene B4 receptor BLT1 were identified as selective cellular receptors for RvE1 action.8,40 It is likely that ChemR23, rather than BLT1, mediates actions on human platelets, because we find that human platelets display ChemR23 on their surface as determined by FACS using an antihuman ChemR23 monoclonal antibody (n = 3; data not shown). Human platelets do not express BLT1 at either the message or surface levels.41 RvE1 blocked U46619 platelet activation but did not compete with recombinant thromboxane receptor binding, indicating that RvE1 is not a direct thromboxane receptor antagonist (Figure 6). In addition, we examined the presence of these receptors on the platelet precursor megakaryocytes, using the MEG-01 cell line,42 and found using FACS that these cells express ChemR23 on their surface without detectable surface levels of BLT1 (n = 4; data not shown). Thus, it is likely that ChemR23 mediates the RvE1 regulation of ADP-stimulated and U46619-stimulated platelets. Further studies are in progress to determine the receptors involved in RvE1 platelet responses that will be reported separately.
The finding that RvE1 blocks both ADP and thromboxane receptor activation of platelets but not platelet activation in response to collagen exposure suggests that RvE1's actions on platelets are specific and point to a role of RvE1 and platelets in promoting the resolution of inflammation and wound healing rather than disrupting physiologic coagulation, where thrombin and collagen are important and likely primary platelet stimuli in vivo.22 The ability of RvE1 to specifically block ADP- and thromboxane-stimulated platelet aggregation may be relevant to the ability of EPA to reduce the incidence of cardiovascular events in humans.4 Also of interest are the recent findings that murine bone marrow produces resolvins such as RvE1 and protectins,43 which suggest in view of the present results that RvE1 may act within bone marrow to quiesce platelet reactivity within the bone marrow compartment or potentially to release RvE1 along with platelets during their maturation and release from the marrow to circulation. In addition, in view of the actions of RvE1 on leukocytes in whole blood (Figures 1,2), it is equally plausible for RvE1 to dampen inappropriate PMN activation within the bone marrow compartment during granulopoiesis. The marrow contains substantial amounts of lipids, and dietary enrichment increases the presence of EPA and DHA as well as their conversion to resolvins and protectins within the bone marrow compartment.43 Hence, the production and presence of RvE1 in bone marrow and its actions on peripheral blood cells and isolated resident macrophages44 suggest that RvE1 plays a critical local signaling role on blood cells that modulates their state and degree of proinflammatory and prothrombotic activity.
In summary, the present results address the systematic actions of RvE1 in whole blood and demonstrate that RvE1 acts directly on leukocytes to modulate adhesion molecules and reduces murine leukocyte rolling in vivo monitored by IVM. Of interest, we also demonstrate for the first time that RvE1 has a direct action on human platelets in PRP, blocking both ADP- and U46619-stimulated platelet aggregation. Together, these results indicate that RvE1 selectively regulates cellular targets in whole blood. The summation of these actions may contribute to the resolution of inflammation as well as to the well-appreciated beneficial impact of aspirin and omega-3 in human cardiovascular health and disease.
Supplementary Material
Acknowledgments
We thank Jeffrey Siegelman for technical assistance, Amiram Ariel for expert advice regarding FACS analysis, and Mary H. Small for expert assistance in paper preparation.
This study was supported in part by National Institutes of Health (NIH, Bethesda, MD) grant nos. GM38765 and DK074448, and by the Specialized Center for Research P50-DE016191 (all to C.N.S.), and, in part, by the Leukocyte Trafficking Core of the Harvard Skin Disease Research Center (Boston, MA; NIH P30 AR42689; U.H.A.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.D. acquired and analyzed data, performed statistical analysis, and contributed to paper preparation; G.F. designed experiments, acquired and analyzed data, performed statistical analysis, and contributed to paper preparation; J.M.S. designed experiments and acquired, analyzed, and interpreted data; N.C. acquired, analyzed, and interpreted data, performed statistical analysis, and contributed to preparation of the paper; M.A. analyzed and interpreted data; A.G. designed video microscopy experiments and acquired and analyzed data; G.C. designed intravital microscopy experiments and acquired, analyzed, and interpreted data; U.H.A. designed intravital microcirculation experiments and contributed to paper preparation; and C.N.S. conceived and designed the research and experiments, analyzed and interpreted results, supervised the research, and contributed to writing the paper.
Conflict-of-interest disclosure: C.N.S. is inventor on patents assigned to Brigham and Women's Hospital on the resolvins and their analogs that are licensed for clinical development and are the subject of consulting agreements. The remaining authors declare no competing financial interests.
Correspondence: Charles N. Serhan, Center for Experimental Therapeutics and Reperfusion Injury, Thorn Building, 7th Floor, Brigham and Women's Hospital and Harvard Medical School, 75 Francis Street, Boston, MA 02115; e-mail: cnserhan@zeus.bwh.harvard.edu.
References
- 1.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 3.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial: Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 4.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 5.Harker LA, Kelly AB, Hanson SR, et al. Interruption of vascular thrombus formation and vascular lesion formation by dietary n-3 fatty acids in fish oil in nonhuman primates. Circulation. 1993;87:1017–1029. doi: 10.1161/01.cir.87.3.1017. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas-Stapleton EH, Lu Y, Hong S, et al. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator Resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. http://www.plosone:org/article/info:doi/10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed]
- 10.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasturk H, Kantarci A, Ohira T, et al. RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 13.Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 14.Bannenberg GL, Chiang N, Ariel A, et al. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 15.Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Schmaier AH. Platelet aggregation testing in platelet-rich plasma: description of procedures with the aim to develop standards in the field. Am J Clin Pathol. 2005;123:172–183. doi: 10.1309/y9ec-63rw-3xg1-v313. [DOI] [PubMed] [Google Scholar]
- 17.Chiang N, Kan WM, Tai HH. Site-directed mutagenesis of cysteinyl and serine residues of human thromboxane A2 receptor in insect cells. Arch Biochem Biophys. 1996;334:9–17. doi: 10.1006/abbi.1996.0423. [DOI] [PubMed] [Google Scholar]
- 18.Majno G, Joris I. Cambridge, MA: Blackwell Science; 1996. Cells, Tissues and Disease: Principles of General Pathology. [Google Scholar]
- 19.Butcher EC. Leukocyte endothelial cell migration: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 20.Strausbaugh HJ, Green PG, Lo E, et al. Painful stimulation suppresses joint inflammation by inducing shedding of L-selectin from neutrophils. Nat Med. 1999;5:1057–1061. doi: 10.1038/12497. [DOI] [PubMed] [Google Scholar]
- 21.DeLeo FR, Renee J, McCormick S, et al. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus AJ. Stratton lecture 1989: thrombosis and inflammation as multicellular processes: pathophysiologic significance of transcellular metabolism. Blood. 1990;76:1903–1907. [PubMed] [Google Scholar]
- 23.Serhan CN, Gotlinger K, Hong S, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 24.Braden GA, Knapp HR, FitzGerald GA. Suppression of eicosanoid biosynthesis during coronary angioplasty by fish oil and aspirin. Circulation. 1991;84:679–685. doi: 10.1161/01.cir.84.2.679. [DOI] [PubMed] [Google Scholar]
- 25.Harris WS, Reid KJ, Sands SA, Spertus JA. Blood omega-3 and trans fatty acids in middle-aged acute coronary syndrome patients. Am J Cardiol. 2007;99:154–158. doi: 10.1016/j.amjcard.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Walcheck B, Kahn J, Fisher JM, et al. Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature. 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- 27.Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR. Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest. 1993;91:651–660. doi: 10.1172/JCI116245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 29.DeForge LE, Kenney JS, Jones ML, Warren JS, Remick DG. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood: separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992;148:2133–2141. [PubMed] [Google Scholar]
- 30.Hartman DA, Ochalski SJ, Carlson RP. The effects of antiinflammatory and antiallergic drugs on cytokine release after stimulation of human whole blood by lipopolysaccharide and zymosan A. Inflamm Res. 1995;44:269–274. doi: 10.1007/BF02032567. [DOI] [PubMed] [Google Scholar]
- 31.Duffield JS, Hong S, Vaidya V, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 32.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knapp HR, Reilly IAG, Alessandrini P, FitzGerald GA. In vivo indexes of platelet and vascular function during fish-oil administration in patients with atherosclerosis. N Engl J Med. 1986;314:937–942. doi: 10.1056/NEJM198604103141501. [DOI] [PubMed] [Google Scholar]
- 34.Needleman P, Whitaker MO, Wyche A, Watters K, Sprecher H, Raz A. Manipulation of platelet aggregation by prostaglandins and their fatty acid precursors: pharmacological basis for a therapeutic approach. Prostaglandins. 1980;19:165–181. doi: 10.1016/0090-6980(80)90163-x. [DOI] [PubMed] [Google Scholar]
- 35.Von Schacky C, Weber PC. Metabolism and effects on platelet function of the purified eicosapentaenoic and docosahexaenoic acids in humans. J Clin Invest. 1986;76:2446–2450. doi: 10.1172/JCI112261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982;69:1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci U S A. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filep JG, Zouki C, Petasis NA, Hachicha M, Serhan CN. Anti-inflammatory actions of lipoxin A4 stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood. 1999;94:4132–4142. [PubMed] [Google Scholar]
- 39.Filep JG, Khreiss T, József L. Lipoxins and aspirin-triggered lipoxins in neutrophil adhesion and signal transduction. Prostaglandins Leukot Essent Fatty Acids. 2005;73:257–262. doi: 10.1016/j.plefa.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 41.Dasari VR, Jin J, Kunapuli SP. Distribution of leukotriene B4 receptors in human hematopoietic cells. Immunopharmacology. 2000;48:157–163. doi: 10.1016/s0162-3109(00)00201-0. [DOI] [PubMed] [Google Scholar]
- 42.Ogura M, Morishima Y, Ohno R, et al. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood. 1985;66:1384–1392. [PubMed] [Google Scholar]
- 43.Poulsen RC, Gotlinger KH, Serhan CN, Kruger MC. Identification of inflammatory and pro-resolving lipid mediators in bone marrow and their profile alteration with ovariectomy and omega-3 intake. Am J Hematol. doi: 10.1002/ajh.21170. Prepublished on February 13, 2008, as DOI 10.1002/ajh.21170. [DOI] [PubMed] [Google Scholar]
- 44.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.