Abstract
An autolysin obtained from culture fluid of Staphylococcus aureus strain 8507 was purified 3,000-fold. One milligram of this preparation (S-5DL) will solubilize 12 mg of cell wall in 1 hr. The major activity is N-acetylmuramyl-l-alanine amidase. Recovery of lytic activity in the purified preparation was repeatably only 20% of the starting level. This suggests that other cell wall lytic enzymes may be present in the starting material. The S-5DL enzyme has been compared to freeze-thaw extracted enzyme (AFZ). Both enzymes precipitate in 0.01 m KPO4 (pH 6.0) and dissolve in 0.1 to 0.7 m NaCl. Fifty per cent of the AFZ activity and 66% of the S-5DL activity bind rapidly to cell walls of S. aureus at 0 C in the presence of magnesium ion. None of the AFZ activity and 66% of the S-5DL activity bind to cell walls at 0 C in the absence of magnesium ion. The cell walls of nine different strains of S. aureus were compared for level of native autolysin activity. These same walls after inactivation of the native autolysin were tested for susceptibility to the S-5DL enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Lache M., Leyh-Bouille M. Structure of the cell walls of Micrococcus lysodeikticus. 3. Isolation of a new peptide dimer, N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanyl-N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanine. Biochemistry. 1968 Apr;7(4):1450–1460. doi: 10.1021/bi00844a030. [DOI] [PubMed] [Google Scholar]
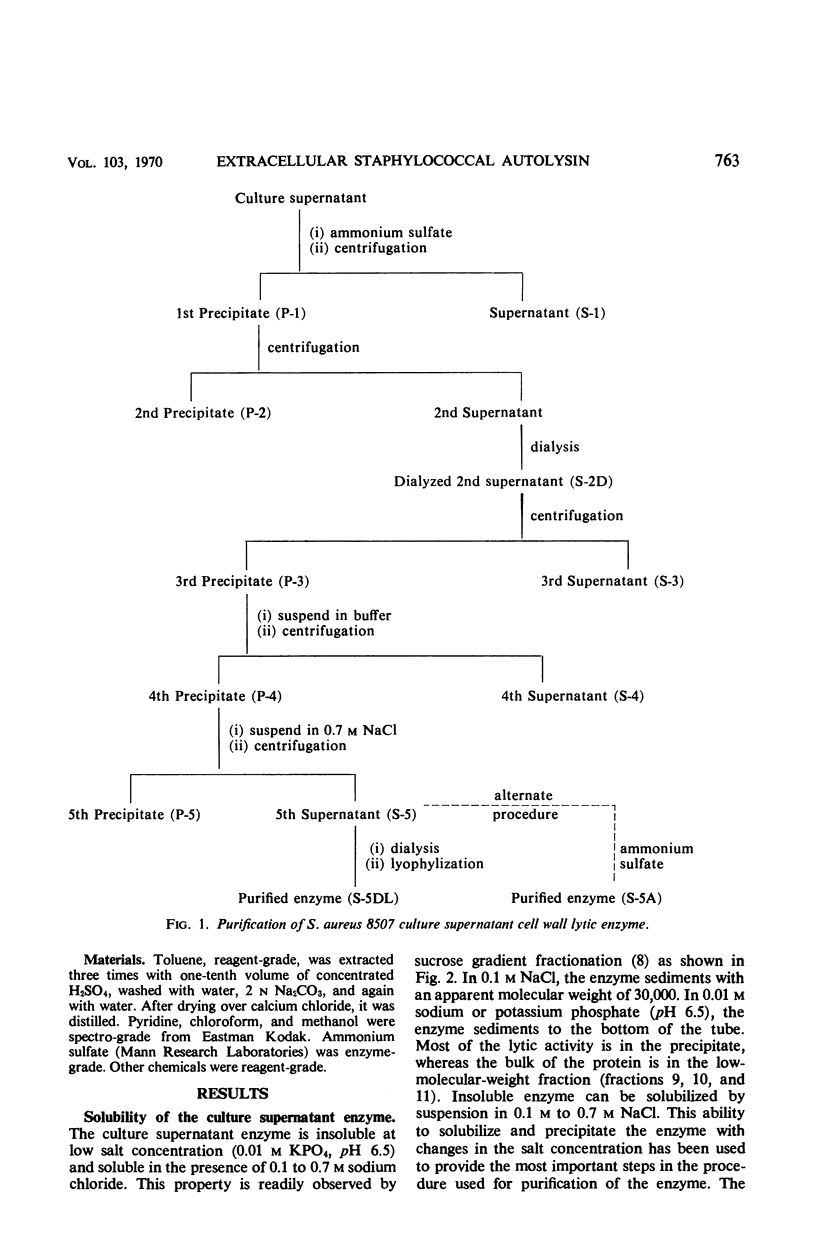
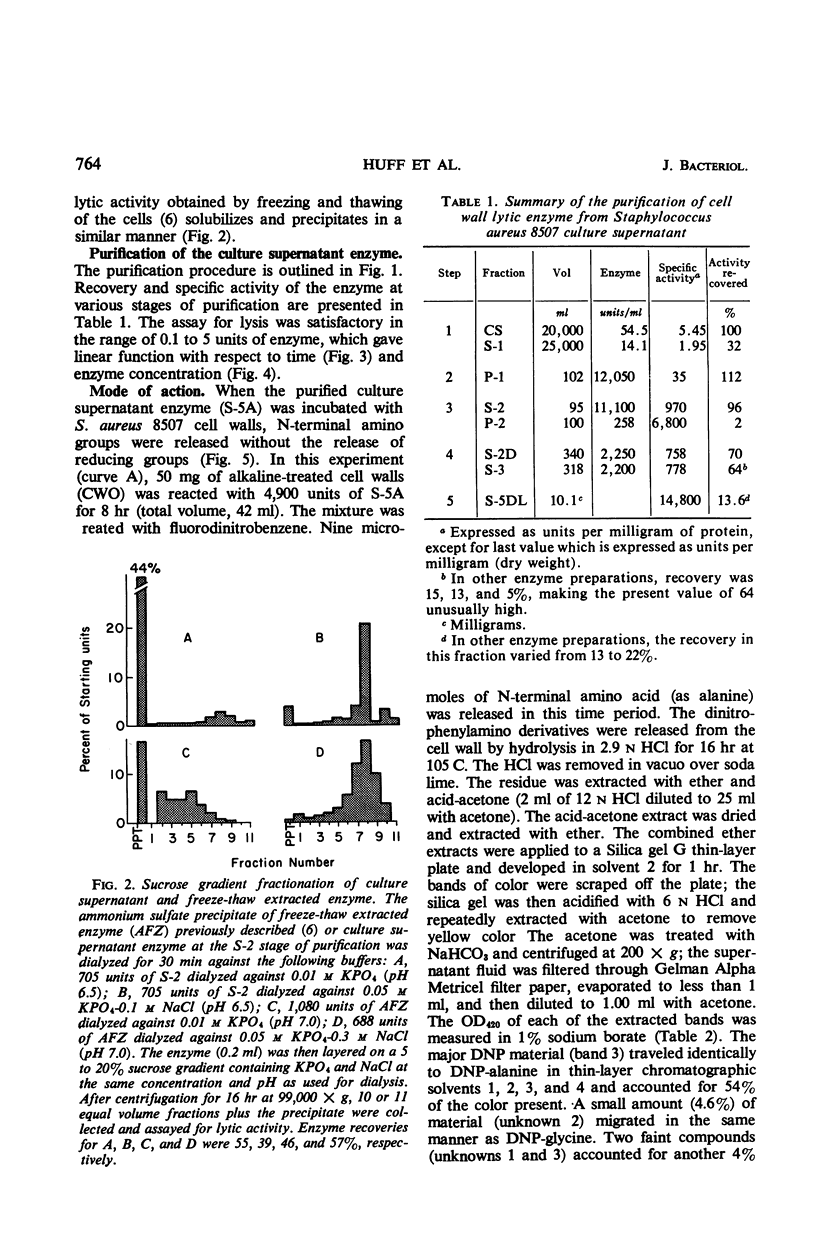
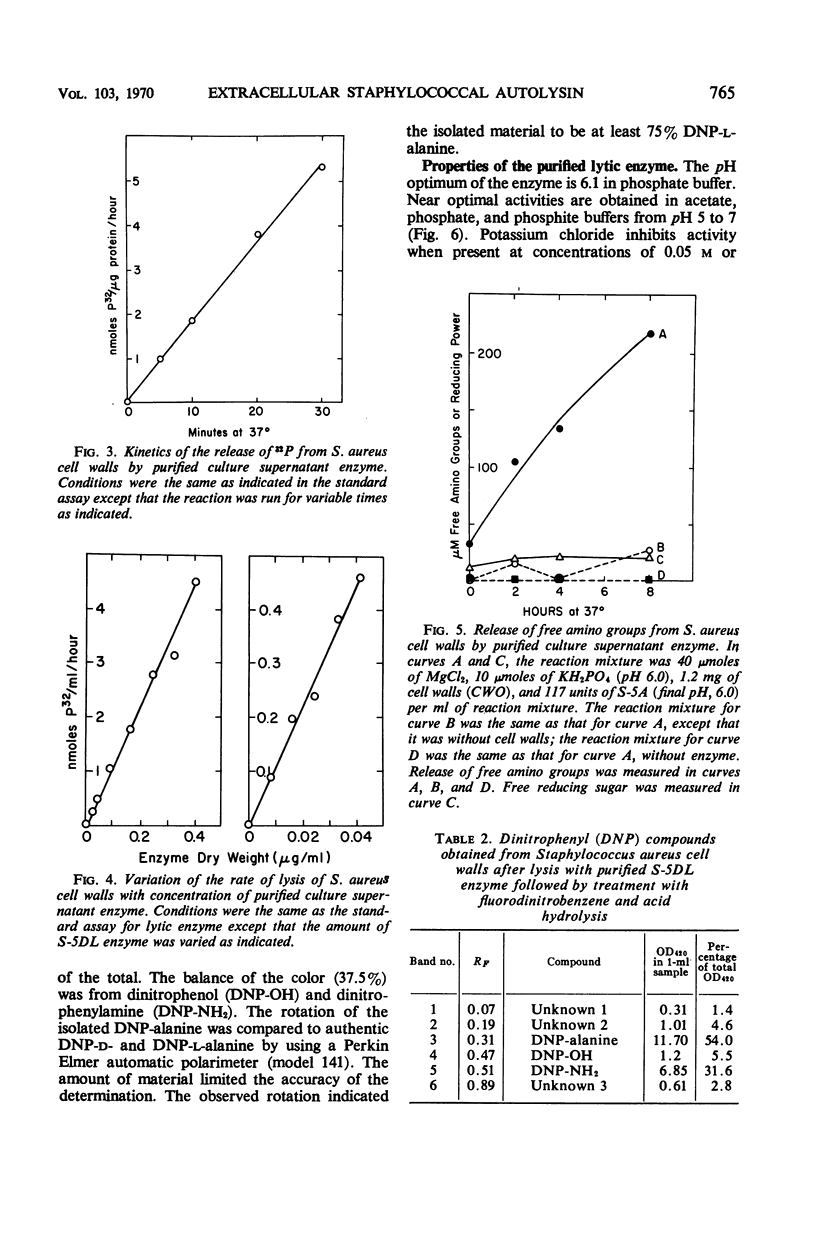
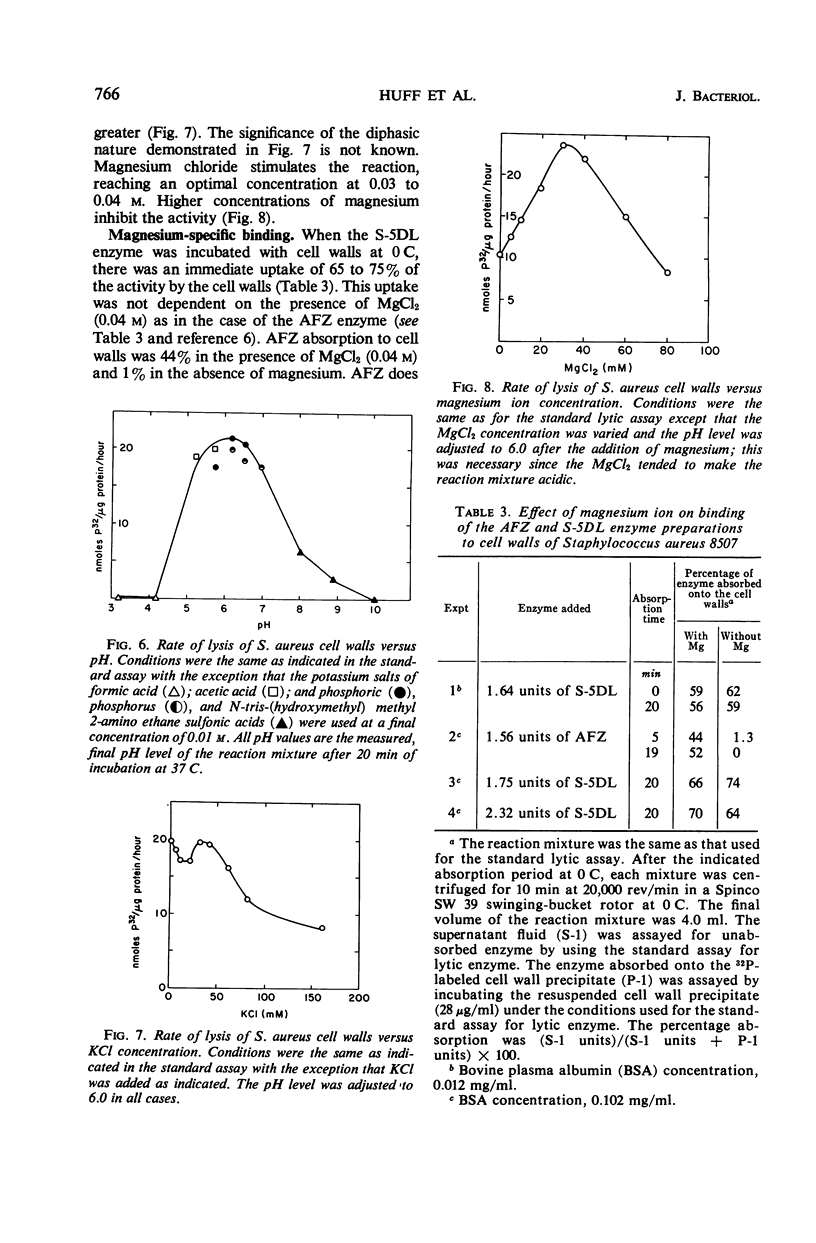
- Huff E., Silverman C. S. Lysis of Staphylococcus aureus cell walls by a soluble staphylococcal enzyme. J Bacteriol. 1968 Jan;95(1):99–106. doi: 10.1128/jb.95.1.99-106.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Micrococcus lysodeikticus: a new type of cross-linkage of the murein. Biochem Biophys Res Commun. 1967 Sep 27;28(6):965–972. doi: 10.1016/0006-291x(67)90074-5. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Kashiba S., Amano T., Kotani S., Imanishi T. Purification and properties of a staphylolytic factor produced by a strain of Staphylococcus epidermidis. Biken J. 1967 Sep;10(3):109–120. [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L., Ensign J. C. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. VII. Mode of action of the bacteriolytic peptidase from Myxobacter and the isolation of intact cell wall polysaccharides. Biochemistry. 1967 Mar;6(3):906–920. doi: 10.1021/bi00855a035. [DOI] [PubMed] [Google Scholar]
- Virgilio R., González C., Muñoz N., Mendoza S. Electron microscopy of Staphylococcus aureus cell wall lysis. J Bacteriol. 1966 May;91(5):2018–2024. doi: 10.1128/jb.91.5.2018-2024.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



