Abstract
Both Akt and Aurora A kinase have been shown to be important targets for intervention for cancer therapy. We report here that Compound A (A-443654), a specific Akt inhibitor, interferes with mitotic progression and bipolar spindle formation. Compound A induces G2/M accumulation, defects in centrosome separation, and formation of either monopolar arrays or disorganized spindles. On the basis of gene expression array studies, we identified Aurora A as one of the genes regulated transcriptionally by Akt inhibitors including Compound A. Inhibition of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, either by PI3K inhibitor LY294002 or by Compound A, dramatically inhibits the promoter activity of Aurora A, whereas the mammalian target of rapamycin inhibitor has little effect, suggesting that Akt might be responsible for up-regulating Aurora A for mitotic progression. Further analysis of the Aurora A promoter region indicates that the Ets element but not the Sp1 element is required for Compound A-sensitive transcriptional control of Aurora A. Overexpression of Aurora A in cells treated with Compound A attenuates the mitotic arrest and the defects in bipolar spindle formation induced by Akt inhibition. Our studies suggest that that Akt may promote mitotic progression through the transcriptional regulation of Aurora A.
Introduction
The Akt protein plays a critical role in preventing cells from undergoing apoptosis [1]. Akt is a serine/threonine kinase originally identified as a cellular homolog of the viral oncogene Akt8. The three isoforms of Akt (Akt1/PKBα, Akt2/PKBβ, and Akt3/PKBγ) share a high degree of structural similarity and sequence homology. The current model suggests that Akt is activated through the phosphatidylinositol 3-kinase (PI3K) pathway on growth factor stimulation. The products of PI3K, especially phosphatidyl inositol (3,4,5) triphosphate, bind to the Pleckstrin homology domain of Akt and target Akt to the plasma membrane where it is phosphorylated on two key residues: Thr308 in the activation loop by PDK1 [2] and Ser473 in the hydrophobic motif of the C-terminal tail by putative PDK2. Proposed candidates of PDK2 include PDK1 [3], integrin-linked kinase [4], Akt itself [5], DNA-PKcs [6], and recently, the mammalian target of rapamycin-rictor complex [7]. Phosphorylation on both Thr308 and Ser473 is required for full activation of Akt [1,2, and the references therein].
Several substrates for Akt have been identified, including Bad, caspase-9, forkhead transcription factors, IκB kinase kinase, glycogen synthase kinase 3 (GSK3), MDM2, p21cip1/WAF1, TSC2, and so on [1,8]. Among these, Bad, caspase-9, and forkhead transcription factors facilitate apoptosis, and the phosphorylation by Akt abolishes their proapoptotic activities [1,8].
PI3K-Akt transduces mitogenic signals from growth factors and promotes G1/S transition. Through multiple mechanisms, Akt downregulates p27, an important Cdk inhibitor that halts cells in lateG1 until cells are ready for DNA synthesis [9–11]. In addition, the PI3K-Akt pathway also regulates the transition at G2/M. Either PI3K inhibitors or the absence of Akt in Akt1-null ES cells were reported to induce a delay in G2/M transition [12–14]. The PI3K-Akt pathway has been shown to regulate mitotic entry in addition to its mitogenic functions at the G1/S transition. Inhibition of PI3K results in a delay in the progression through G2/M, which can be rescued by overexpressing Akt [12–14]. PTEN-null ES cells were shown to transit faster through the G2/M phase [12]. Overexpressing a dominant-negative mutant of Akt also arrests cells in G2/M [15]. Finally, PI3K-Akt pathway regulates mitotic entry through controlling the timing of Cdc2 activation [16].
Wee1 and Myt1 are two kinases that phosphorylate Cdc2 at Thr14/Tyr15 and inhibit Cdc2 kinase activity. Akt phosphorylates and downregulates Myt1 at the G2/M boundary [17]. In addition, Akt was shown to phosphorylate WEE1Hu at Ser642, which in turn provides the binding site for 14-3-3θ. This 14-3-3θ binding translocates WEE1Hu into the cytoplasm and, thus, prevents its inhibitory phosphorylation on Cdc2 [18]. Akt also prevents Plk1 degradation through CHFR and promotes mitotic entry under normal conditions and after DNA damage [19].
Aurora kinases are serine/threonine kinases that regulate mitotic events, ranging from centrosome maturation, mitotic spindle formation, chromosome segregation to cytokinesis [20–23]. The three members of Aurora kinase family in metazoans share extensive structure and sequence similarities [20–23]. However, they show distinct localizations and functions during mitosis. Aurora A localizes to centrosomes and is essential for centrosome duplication and maturation [20–23]. Overexpression of Aurora A leads to genomic instability and neoplastic transformation, demonstrating that Aurora A is a bonafide oncogene [24–26]. Cells depleted of Aurora A by siRNA are arrested at mitosis [27,28] and display a G2 delay in synchronized cells [28]. Aurora B is localized to centromeres in early mitosis, relocates to the central spindle in anaphase and the spindle midzone during telephase, and finally migrates to the midbody during cytokinesis [20–22,29]. Aurora B functions as a chromosome passenger protein involved in chromosome condensation, kinetochore-microtubule attachment, chromosome alignment in metaphase, and midbody function during cytokinesis [20–22]. Aurora C is also associated with the centrosomes, but its function in mitosis is not well defined [21,22].
We have previously identified a potent and selective Akt inhibitor, hereafter referred to as Compound A (A-443654) [30,31]. Here, we show that Compound A induces mitotic arrest and defects in spindle formation in cells, consistent with an Aurora A-deficient phenotype, whereas its enantiomer (Compound B) does not. Akt inhibition was found to down-regulate Aurora A expression. Overexpression of Aurora A rescues the mitotic defect induced by Akt inhibition. Our data suggest a novel mechanism in which Akt promotes mitotic progression through the transcriptional regulation of Aurora A.
Materials and Methods
Cell Lines Agents
All chemicals were purchased from Sigma (St. Louis, MO). H1299, MiaPaca-2, and HeLa cells were obtained from American Type Culture Collection (Manassas, VA).
Plasmids
The 1.8-kb DNA fragment corresponding to -1486 to +355 of the 5′-flanking region of Aurora A gene [32] was polymerase chain reaction amplified from genomic DNA isolated from normal human fibroblast using the Qiagen genomic DNA isolation kit (Qiagen, Valencia, CA). The fragment was gel-purified and cloned into the BglII site of pGL3-basic (Promega, Madison, WI) to obtain pGL-1.8kb. Various constructs were subcloned from pGL-1.8kb. For pCDNA.3. Aurora A, polymerase chain reaction fragment-encoded Aurora A was cloned between the BamHI and XhoI sites in pcDNA3.1/myc-His A (Invitrogen, Carlsbad, CA). The resulting construct encodes Aurora A with both a myc tag and a polyhistidine tag at the C-terminus. All the inserted DNA fragments and generated mutations were confirmed by sequencing.
Cell Transfection and Luciferase Assay
H1299 cells in a density of 1 x 104 per well in 96-well black plates (Cat# 7716-2380; Whatman, Clifton, NJ) were transiently transfected with 0.3 µg of various plasmids using Lipofectamine 2000 (Cat# 11668-027; Invitrogen). Luminescence was determined using Steady-Glo Reagent (Cat# E2510; Promega) according to the manufacturer's protocol.
Immunofluorescence
Cells were cultured in Lab-Tek-2 chamber slides (Cat# 155382; Nalge Nunc International, Rochester, NY) at 4 x 104 per chamber. After incubation with Compound A or B for 24 hours, the cells were fixed and permeabilized with methanol/acetone (50:50) for 20 minutes and blocked with a blocking solution (3% bovine serum albumin in PBS) for another 20 minutes. The cells were then incubated sequentially with the following antibodies for 2 hours in a blocking buffer with three times of washes in between: rabbit polyclonal anti-γ-tubulin antibody (at 1:500, Cat# T3559; Sigma), donkey antirabbit IgG(H+L) conjugated with Alexa Fluor 555 (at 1:500, Cat# A31572; Invitrogen), and monoclonal anti-α-tubulin-fluorescein isothiocyanate antibody (at 1:100, Cat# F2168; Sigma). Finally, the cells were covered with mounting medium Prolong Gold antifade reagent with DAPI (Cat# P36935; Invitrogen), sealed with coverslips, counted, and photographed with a microscope (Axiovert 200M; Carl Zeiss, Inc, Chester, VA). All the procedures were performed at room temperature.
Flow Cytometry Analysis
Cells were harvested by pooling attached and detached cells and pelleted by centrifugation at 800g for 5 minutes at 4°C. The cells were washed with PBS and resuspended in 0.5 ml of ice-cold staining solution (5 µg/ml propidium iodide, 40 U/ml RNase A, 0.5% Triton X-100, in PBS). After 1 hour at 4°C in the dark, the DNA content was analyzed using a Beckton Dickinson ExCalibur Flow Cytometer (San Jose, CA).
Western Blot Analysis
Cells were harvested and lysed in buffer B (20 mM HEPES, pH 7.5, 10 mM NaCl, 20 mM NaF, 1 mM EDTA, 1 mM EGTA, 5 mM sodium pyrophosphate, 2 mM sodium vanadate, 10 mM β-glycerophosphate, and 1% NP-40) on ice for 30 minutes. The samples were centrifuged at 12,000g at 4°C for 10 minutes. The supernatants were used as cell extracts. Rabbit anti-Aurora A, anti-Aurora B, and anti-histone H3 antibodies were purchased from Cell Signaling Technology, Inc (Beverly, MA). Anti-actin, anti-PLK1, and anti-cyclin B1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Microarray Analysis
Total RNA was extracted from MiaPaca-2 cells treated with inhibitors for 5 hours (n = 2 for each treatment). The total RNA were intact as judged by Agilent 2100 analysis (Agilent Technologies, Santa Clara, CA). Approximately 8 µg of total RNA from each sample was used to prepare biotin-labeled cRNA target using standard Affymetrix protocols. The Affymetrix Human chip U133Av2 (Affimetrix, Santa Clara, CA) was used, and 10 µg of cRNA target was applied to each array. Scanned images were loaded into the Rosetta Resolver 4.0 database (Rosetta Biosoftware, Seattle, WA) and processed using the Resolver Affymetrix error model. The replicates (n = 2) of drug-treated samples were informatically combined within Resolver and ratios constructed relative to the combined DMSO controls. A combination of classification, clustering, gene ontology, and pathway mapping analyses were used to assess the function of the regulated genes.
Results
Inhibition of Akt Results in Mitotic Arrest
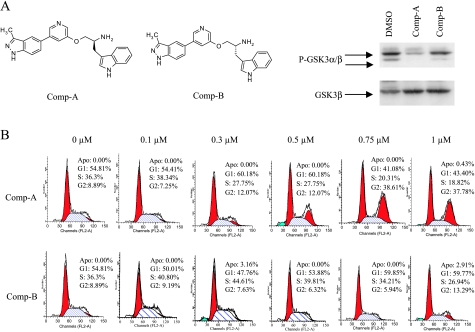
Compound A is a potent and selective Akt inhibitor with a Ki of 160 pM against Akt1, and it is equally potent against Akt2 and Akt3 in cells. Compound B, the enantiomer of Compound A, is much less active than Compound A against Akt but has very similar activities against other kinases (Figure 1A) [30,31]. Compound A inhibits Akt in H1299 cells at 0.6 µM as demonstrated by its ability to inhibit the phosphorylation of GSK3α/β, whereas Compound B does not, and thus, Compound B provides a control for Compound A (Figure 1A) [30,31]. Similar concentrations of Compound A induced G2/M accumulation in H1299 cells, whereas compound B did not, suggesting that the G2/M accumulation is due to Akt inhibition (Figure 1B). Similar G2/M accumulation was also observed with other Akt inhibitors such as Compound C [31] (data not shown) or in other cell lines regardless whether the cells have wild type p53 (HCT116 and RKO) or have defective p53 functions (RKO-E6, MiaPaCa, and HeLa; data not shown). Compound A is very selective and only inhibits mitotic kinases at very high concentrations. The selectivity compared to its activity toward Akt are at least 3800-fold for Aurora A, Aurora B, Plk1, Plk3, and Plk4. Its selectivity against Cdc2 versus Akt is 280-fold. Therefore, it is unlikely that the G2/M accumulation induced by Compound A is due to a direct inhibition of mitotic kinases.
Figure 1.
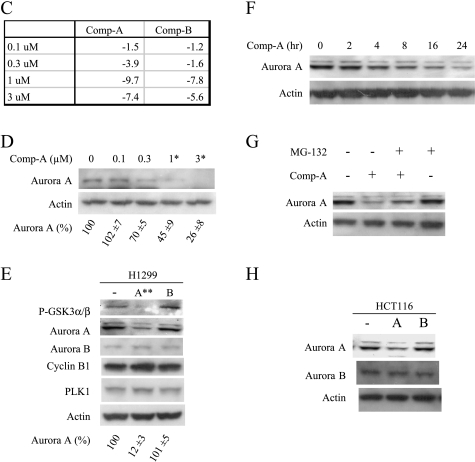
Akt inhibition down-regulates Aurora A and induces mitotic arrest. (A) Western analysis of P-GSK3α/β and total GSK3α/β in H1299 cells treated with Compound A or B at 0.6 µM concentration for 2 hours. (B) FACS analysis of H1299 cells treated with Compound A or B for 24 hours. (C) MiaPaca-2 cells were treated with Compound A or B at the indicated concentrations for 5 hours. Total RNA was isolated and subjected to microarray analysis as described. The fold changes compared to the DMSO control in Aurora A mRNA levels were listed. (D) MiaPaca-2 cells were treated with Compound A at the indicated concentrations for 24 hours. The cells were harvested, and Western blot analysis was carried out. Aurora A levels were quantified from three experiments using GS-800 calibrated densitometer (Bio-Rad, Hercules, CA) and listed beneath each western gels. Student's t test were performed and *P < .05 or **P < .01 is obtained between the control and the marked lane. (E) H1299 cells were treated with Compound A or B at 0.6 µM for 24 hours. The cells were harvested, and Western analysis was carried out. Statistical analysis was done as in (D). (F) H1299 cells were treated with Compound A at 0.6 µM for the indicated times. The cells were harvested, and Western analysis was carried out. (G) H1299 cells were treated with Compound A at 0.6 µM in the presence or absence of 20 µM MG132 for 24 hours. The cells were harvested, and Western analysis was carried out. (H) HCT116 cells were treated with Compound A or B at 0.5 µM for 24 hours. The cells were harvested, and Western analysis was carried out.
Inhibition of Akt Reduces the mRNA and Protein Levels of Aurora A
To explore the mechanism of mitotic regulation by Akt, we carried out microarray experiments with Compounds A and B and identified Aurora A as one of the genes regulated by Akt. Aurora A mRNA levels were significantly reduced when Akt was inhibited in cells by Compound A but not by Compound B at 0.3 µM in MiaPaca-2 cells, the concentration at which Akt is inhibited by Compound A in this cell line [30,31] (Figure 1C). Aurora A kinase is one of the nine genes that showed dose-dependent regulation by Compound A between 0.1 and 0.3 µM, whereas no genes showed dose-dependent regulation by Compound B within the same concentration range (data not shown). This suggests that Aurora A kinase is one of the most prominently regulated genes by Akt. The protein levels of Aurora A were also reduced in the cells treated with Compound A in a concentration-dependent manner in MiaPaca-2 (Figure 1D). In H1299 cells, Compound A reduced the protein level of Aurora A but not other mitotic proteins including Aurora B, PLK1, and cyclin B1 (Figure 1E). Compound A reduced the protein level of Aurora in a time-dependent manner (Figure 1F). Inclusion of MG132 inhibited Compound A-medicated reduction of Aurora A, indicating the involvement of proteasome pathway in the process (Figure 1G). Similar inhibition of Aurora A by Compound A was also observed in HeLa cells at the same concentration that induces G2/M accumulation (data not shown). Compound A-mediated reduction of Aurora A was independent of the status of p53, because Compound A showed the same effect in HCT116 cells which has a wild type p53 (Figure 1H).
Akt Regulates the Promoter Activity of Aurora A
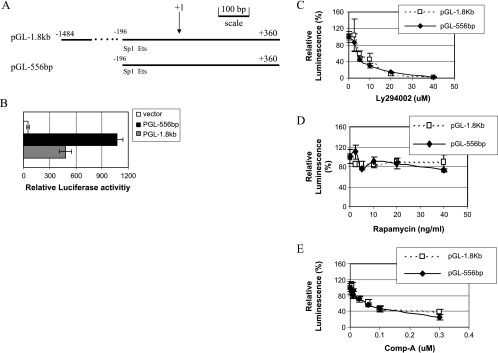
We cloned the Aurora A promoter region corresponding to -1486 to +355 of the 5′-flanking sequence into a luciferase reporter vector pGL3 [32] and assigned it as pGL-1.8kb. pGL-556bp, a truncation of pGL-1.8kb containing the Sp1 and Ets elements, was also generated (Figure 2A). Transient transfection experiment in H1299 cells showed that both constructs had high levels of promoter activity (Figure 2B). In fact, pGL-556bp showed better activity than pGL-1.8kb, indicating that there may be an inhibitory element located in the region corresponding to -1486 to -196 of the Aurora A promoter. The luciferase activities from both pGL-1.8kb and pGL-556bp were inhibited by LY294002 (Figure 2C) and Compound A (Figure 2E) in a concentration-dependent manner, whereas rapamycin had little effect (Figure 2D).
Figure 2.
Identification of the Akt-response element in Aurora A promoter. (A) Schematic representation of the Aurora A promoter-luciferase constructs. (B) H1299 cells were transiently transfected with pGL-1.8kb or pGL-556bp, and the luciferase activities were assessed 24 hours after transfection. (C, D, and E) H1299 cells were transfected with pGL-1.8kb and pGL-556bp. Approximately 8 hours later, LY294002 (C), rapamycin (D), or Compound A (E) was added, and the luciferase activities were assessed 24 hours later.
Akt Regulates Aurora A Expression through the Ets Element
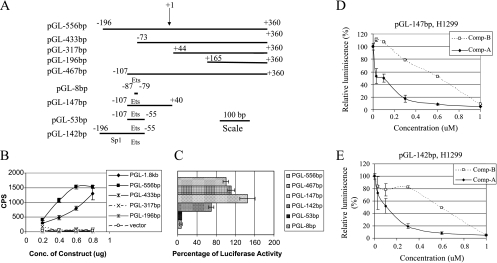
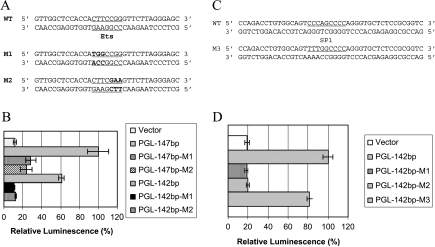
To identify the transcription element that is responsible for the Akt-mediated regulation of Aurora A, a series of truncated constructs were generated (Figure 3A). The Ets element is necessary for the activity but is not sufficient because pGL-53bp and pGL-8bp lost the activity. It needs a longer length either at 5′ or 3′ end for full activity, which may reflect a requirement for a sufficient space for transcription factor binding. The shortest fragments that retained most of the activity are -107 ∼+40 or -196 ∼-55 in pGL-147bp or pGL-142bp, respectively (Figure 3, B and C). The Sp1 site, however, is not necessary because pGL-147bp retained most of the activity (Figure 3C). The luciferase activities from pGL-147bp and pGL-142bp can be inhibited by Compound A (Figure 3, D and E). Compound A inhibited 91% and 92% of the luciferase activity of pGL-147bp and pGL-142bp, respectively, at the concentration of 0.6 µM (Figure 3, D and E). At 0.6 µM, although Compound B inhibited 45% and 51% of the luciferase activity of pGL-147bp and pGL-142bp, respectively (Figure 3, D and E), this was not sufficient for Aurora A protein reduction (Figure 1E). Therefore, Compound A blocked Aurora A protein expression, whereas Compound B did not at this concentration.
Figure 3.
Identification of Ets as the Akt-response element. (A) Schematic representation of the Aurora A promoter luciferase constructs. (B and C) H1299 cells were transfected with the indicated Aurora A promoter constructs at indicated concentrations. Experiments were done as in Figure 2. Data are representative of three independent experiments. (D and E) H1299 cells were transfected with either pGL-147bp (D) or pGL-142bp (E). Approximately 8 hours later, Compound A or B was added, and the luciferase activities were assessed 24 hours later.
The luciferase activities decreased significantly in four constructs containing the mutations of the Ets element, pGL-147-M1, pGL-147-M2, pGL-142-M1, and pGL-142-M2 (Figure 4, A and B). Conversely, pGL-142-M3 with an Sp1 mutation retained all the activity of wild type pGL-142 (Figure 4, C and D), suggesting that Sp1 is not necessary for such an activity of the Aurora promoter. Similar data were obtained in HeLa cells (data not shown).
Figure 4.
Mutational analysis of Ets and Sp1 elements. (A and C) DNA sequences of the wild type and the mutated Ets or Sp1 sequences. The underlined sequences are either the Ets or the Sp1 sites, and the nucleotides in bold are the mutated nucleotides. (B and D) H1299 cells transfected with the indicated constructs, and luciferase activities were analyzed 24 hours after transfection. Data are representative of three independent experiments.
Akt Inhibition Induces Abnormal Mitosis
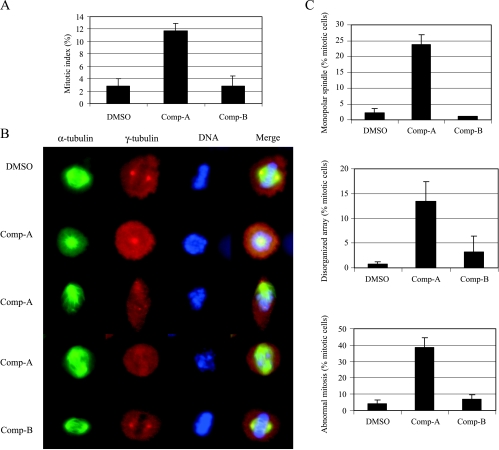
We used H1299 cells for further mitotic phenotype studies because H1299 cells give nice mitotic morphology. Compound A inhibited Akt and induced a significant increase in the mitotic index in H1299 as measured by condensed chromosomes and spindle formation (Figure 5A). We observed that most of the mitotic cells treated with Compound A contained abnormal spindle formation consisting of rosette (second row) or monopolar arrays (third row) instead of normal bipolar spindles as in the control cells (Figure 5B). Bipolar spindles could also form in cells treated with Compound A (fourth row). However, the bipolar spindles were not aligned well and, as in the cells with rosette or monopolar spindles, chromosomes were not aligned at the equators as are those in normal controls (Figure 5B). Quantitative analysis indicated that abnormal spindle formation (monopolar spindles or disorganized array) dramatically increased in Compound A-treated cells (Figure 5C). Therefore, in addition to regulating mitotic entry [12–14], Akt also regulates centrosome separation and spindle formation during premetaphase. Aurora A deficiency results in defects in centrosome separation and biopolar spindle formation [33–35]. The abnormal mitotic phenotypes we observed here with Akt inhibition are consistent with the Aurora A kinase null phenotypes.
Figure 5.
Inhibition of Akt results in abnormal mitosis. H1299 cells were treated with Compound A or B at 0.6 µM for 24 hours. (A) Mitotic index was scored on the basis of chromosome condensation, disappearance of nuclear membrane, and spindle formation. (B) Typical examples of normal and abnormal mitotic cells. (C) Quantitative assessment of normal and abnormal mitotic spindles. For each treatment condition, more than 150 mitotic cells were scored. Data are representative of three independent experiments.
Overexpression of Aurora A Partially Rescues the Mitotic Arrest Induced by Akt Inhibition
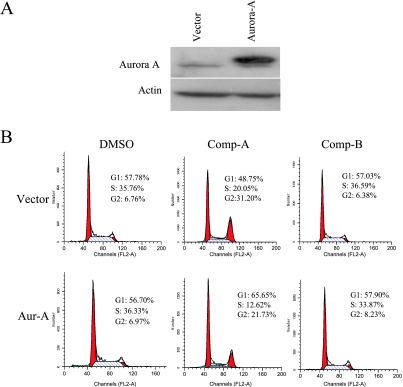
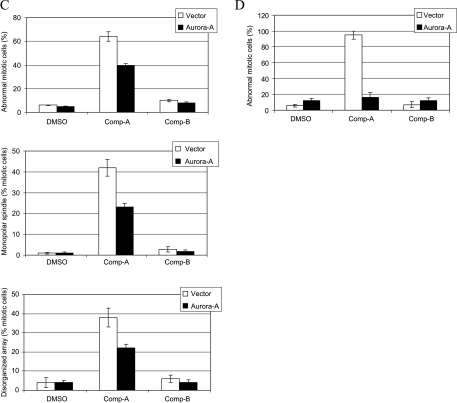
To examine whether Akt inhibition induces mitotic arrest through Aurora A down-regulation, we overexpressed Aurora A to determine whether it could rescue the mitotic arrest induced by Compound A treatment. Aurora A kinase was transiently overexpressed from a CMV promoter using a pcDNA vector, which is not regulated by Akt (Figure 6A). We treated these cells with Compound A and analyzed cell cycle progression. As shown in Figure 6B, G2/M accumulation was significantly reduced in Aurora A-overexpressing cells when compared to that in cells transfected with vector alone after Compound A treatment. In addition, the population of abnormal mitotic cells was also reduced in Aurora A-overexpressing cells (Figure 6C). We estimated that 50% of the cells were transfected by cotransfecting a GFP coding construct (data not shown). In the transfected cell population, the mitotic defect can be reversed by the expression of Aurora A to almost the levels in the vehicle controls (Figure 6D). Therefore, the mitotic defects induced by Akt inhibitor Compound A are consistent with the Aurora A-deficient phenotypes, and these defects were rescued by overexpressing Aurora A. This suggests that Akt may modulate mitotic progression, at least partly, through Aurora A regulation.
Figure 6.
Overexpression of Aurora A rescues the mitotic defects induced by Akt inhibition. (A) H1299 cells were transfected with pcDNA3.1 or pcDNA3-Aurora A. Cell extracts were prepared 24 hours after transfection and were subjected to Western blot analysis. (B and C) H1299 cells were transfected as described in (A). The cells were then treated with DMSO, 0.6 µM Compound A or B for 24 hours. Cells were stained with propidium iodide for FACS analysis or were immunostained with DAPI and antibodies against α-and γ-tubulins. FACS analysis (B) and abnormal mitosis (C) were scored as described. For each condition, more than 150 mitotic cells were scored. Data are average of three independent experiments. (D) Experiment was done as in (C) except 0.8 µg of padtrack-GFP was cotransfected with pcDNA3.1-Aurora A. GFP were immunostained with fluorescein isothiocyanate-conjugated GFP antibody (Abcam, Cambridge, MA). Only GFP-positive cells (60–100 cells) were scored.
Discussion
Aurora A is essential for centrosome maturation, separation, and bipolar spindle formation [23,28,33,35–39]. We have shown that an Akt inhibitor (Compound A) induces a G2/M arrest at a concentration that inhibits Akt in cells [31], whereas its enantiomer (Compound B) at the same concentration does not (Figures 1 and 5). In addition to the defects in mitotic entry reported with PI3K inhibitors or Akt inhibitors in the literature, we observed that a significant portion of those cells was arrested in mitosis (Figure 5A). The presence of abnormal spindles, such as monopolar arrays due to the defect in centrosome separation, or disorganized spindles (Figure 5, B and C) is consistent with the Aurora A defect [33–35,40]. Exogenous expression of Aurora A in cells treated with Compound A rescues the spindle formation defects and the mitotic arrest (Figure 6), suggesting that the mitotic defects induced by Akt inhibition are, at least partly, due to the inability to express Aurora A kinase in cells. Thus, Akt regulates mitotic entry as well as bipolar spindle formation through controlling Aurora A expression. Our data are consistent with the earlier report that an Akt activity blocker, 1L-6-hydroxy-methylchiro-inositol 2-2-O-methyl-3-O-octadecylcarbonate, and the PI3K inhibitor, LY294002, delay mitotic cells progressing into G1 phase of the next cycle [15]. We also tried to strengthen our finding using Akt1 siRNA. Although Akt1 siRNA were able to reduce approximately 70% of Akt1 protein in H1299 cells, it has no effect on the phosphorylation of GSK3 and aurora A (data not shown). This is probably due to the reason that either Akt1 protein level was not reduced enough or Akt2/3 might be able to compensate for the loss of Akt1 efficiently in H1299 cells. In fact, only a small portion of Akt is active in wild type MEF cells, and Akt1 is able to compensate for the loss of Akt3 in its prosurvival activity [41]. Because Compound A is a pan-Akt inhibitor, it is likely that all isoforms of Akt have to be inhibited to see the reduction of Aurora A.
Akt inhibitor (Compound A) interferes with the proper formation of the bipolar spindle during mitosis by controlling the transcription of the Aurora A gene. We showed that the Ets element located in the Aurora A promoter region is necessary but not sufficient for such a regulation. The PI3K-Akt pathway has been shown to positively or negatively regulate various Ets transcription factors depending on the individual Ets factors [42–44]. Further studies are warranted to search for the Ets factor(s) responsible for Akt-directed regulation of Aurora A expression. Interestingly, Akt was shown to phosphorylate CHFR, preventing its potential role in Plk1 degradation [19]. CHFR is also implicated in degradation of Aurora A [45], providing yet another potential venue for Akt to regulate Aurora A protein levels. In addition, overexpression of Aurora A induces the activation of Akt through a p53-dependent manner [46,47], indicating that there is a positive feedback interplay between Akt and Aurora A.
These findings have potential impact on the strategies used in developing Akt inhibitors as therapeutics. Although additional toxicities may be associated with the Aurora A suppression, the benefit of inhibiting Aurora A in tumor cells, especially those that overexpress Aurora A, could supercede the risk of toxicity [48]. Our data also suggest the cancer patients that overexpress Aurora A may serve as a suitable population for using Akt inhibitors in the clinic.
Acknowledgments
The authors thank Joel Leverson and Loren Lakso for technical support with the microscopic analyses.
Abbreviations
- PI3K
phosphatidylinositol 3-kinase
- GSK3
glycogen synthase kinase 3
References
- 1.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes CP, Alessi DR. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 4.Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462–27469. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 5.Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 6.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 9.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 10.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 11.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 12.Kandel ES, Skeen J, Majewski N, Di CA, Pandolfi PP, Feliciano CS, Gartel A, Hay N. Activation of Akt/protein kinase B overcomes a G(2)/M cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 2002;22:7831–7841. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dangi S, Cha H, Shapiro P. Requirement for phosphatidylinositol-3 kinase activity during progression through S-phase and entry into mitosis. Cell Signal. 2003;15:667–675. doi: 10.1016/s0898-6568(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 14.Shtivelman E, Sussman J, Stokoe D. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr Biol. 2000;12:919–924. doi: 10.1016/s0960-9822(02)00843-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee S-R, Park J-H, Park EK, Chung CH, Kang S-S, Bang O-S. Akt-induced promotion of cell cycle progression at G(2)/M phase involves upregulation of NF-Y binding activity in PC12 cells. J Cell Physiol. 2005;205:270–277. doi: 10.1002/jcp.20395. [DOI] [PubMed] [Google Scholar]
- 16.Roberts EC, Shapiro PS, Nahreini TS, Pages G, Pouyssegur J, Ahn NG. Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signaling pathways in somatic cell mitosis. Mol Cell Biol. 2002;22:7226–7241. doi: 10.1128/MCB.22.20.7226-7241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okumura E, Fukuhara T, Yoshida H, Hanada SS, Kozutsumi R, Mori M, Tachibana K, Kishimoto T. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat Cell Biol. 2002;4:111–116. doi: 10.1038/ncb741. [DOI] [PubMed] [Google Scholar]
- 18.Katayama K, Fujita N, Tsuruo T. Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition. Mol Cell Biol. 2005;25:5725–5737. doi: 10.1128/MCB.25.13.5725-5737.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shtivelman E. Promotion of mitosis by activated protein kinase B after DNA damage involves polo-like kinase 1 and checkpoint protein CHFR. Mol Cancer Res. 2003;1:959–969. [PubMed] [Google Scholar]
- 20.Andrews PD. Aurora kinases: shining lights on the therapeutic horizon? Oncogene. 2005;24:5005–5015. doi: 10.1038/sj.onc.1208752. [DOI] [PubMed] [Google Scholar]
- 21.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 22.Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004;14:29–36. doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Ruderman JV. Aurora A, mitotic entry, and spindle bipolarity. Proc Natl Acad Sci USA. 2006;103:5811–5816. doi: 10.1073/pnas.0601425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand S, Penrhyn LS, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Burke T, Dempsey J, Diaz B, Collins E, Toth J, Beckmann R, Ye X. Mitotic requirement for aurora A kinase is bypassed in the absence of aurora B kinase. FEBS Lett. 2005;579:3385–3391. doi: 10.1016/j.febslet.2005.04.080. [DOI] [PubMed] [Google Scholar]
- 28.Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 29.Murata HM, Tatsuka M, Wang YL. Probing the dynamics and functions of aurora B kinase in living cells during mitosis and cytokinesis. Mol Biol Cell. 2002;13:1099–1108. doi: 10.1091/mbc.01-09-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y, Shoemaker AR, Liu X, Woods KW, Thomas SA, de Jong R, Han EK, Li T, Stoll VS, Powlas JA, et al. Potent and selective inhibitors of Akt kinases slow the progression of tumors in vivo. Mol Cancer Ther. 2005;4:977–986. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y, Liu X, Han E-K, Guan R, Shoemaker AR, Oleksijew A, Woods KW, Fisher JP, Klinghofer V, Lasko L, et al. Optimal classes of chemotherapeutics sensitized by specific small molecule inhibitors of Akt in vitro and in vivo. Neoplasia. 2005;7:922–1000. doi: 10.1593/neo.05355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka M, Ueda A, Kanamori H, Ideguchi H, Yang J, Kitajima S, Ishigatsubo Y. Cell-cycle-dependent regulation of human aurora A transcription is mediated by periodic repression of E4TF1. J Biol Chem. 2002;277:10719–10726. doi: 10.1074/jbc.M108252200. [DOI] [PubMed] [Google Scholar]
- 33.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–51795. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 34.Giet R, Prigent C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J Cell Sci. 1999;112:3591–3601. doi: 10.1242/jcs.112.21.3591. [DOI] [PubMed] [Google Scholar]
- 35.Giet R, Prigent C. The Xenopus laevis aurora/Ip11p-related kinase pEg2 participates in the stability of the bipolar mitotic spindle. Exp Cell Res. 2000;258:145–151. doi: 10.1006/excr.2000.4903. [DOI] [PubMed] [Google Scholar]
- 36.Marumoto T, Zhang D, Saya H. Aurora-A—a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 37.Giet R, McLean D, Descamps S, Lee Michael J, Raff-Jordan W, Prigent C, Glover DM. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J Cell Biol. 2002;156:437–451. doi: 10.1083/jcb.200108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–1116. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giet R, Uzbekov R, Cubizolles F, Le Guellec K, Prigent C. The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J Biol Chem. 1999;274:15005–15013. doi: 10.1074/jbc.274.21.15005. [DOI] [PubMed] [Google Scholar]
- 40.Gadea BB, Ruderman JV. Aurora kinase inhibitor ZM447439 blocks chromosome-induced spindle assembly, the completion of chromosome condensation, and the establishment of the spindle integrity checkpoint in Xenopus egg extracts. Mol Biol Cell. 2005;16:1305–1318. doi: 10.1091/mbc.E04-10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Shi Y, Birnbaum MJ, Ye K, De Jong R, Oltersdorf T, Giranda VL, Luo Y. Quantitative analysis of anti-apoptotic function of Akt in Akt1 and Akt2 double knock-out mouse embryonic fibroblast cells under normal and stressed conditions. J Biol Chem. 2006;281:31380–31388. doi: 10.1074/jbc.M606603200. [DOI] [PubMed] [Google Scholar]
- 42.Smith JL, Schaffner AE, Hofmeister JK, Hartman M, Wei G, Forsthoefel D, Hume DA, Ostrowski MC. ets-2 is a target for an Akt (protein kinase B)/Jun N-terminal kinase signaling pathway in macrophages of motheatenviable mutant mice. Mol Cell Biol. 2000;20:8026–8034. doi: 10.1128/mcb.20.21.8026-8034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galetic I, Maira SM, Andjelkovic M, Hemmings BA. Negative regulation of ERK and Elk by protein kinase B modulates c-fos transcription. J Biol Chem. 2003;278:4416–4423. doi: 10.1074/jbc.M210578200. [DOI] [PubMed] [Google Scholar]
- 44.Figueroa C, Vojtek AB. Akt negatively regulates translation of the ternary complex factor Elk-1. Oncogene. 2003;22:5554–5561. doi: 10.1038/sj.onc.1206496. [DOI] [PubMed] [Google Scholar]
- 45.Yu X, Minter DK, Malureanu L, Zhao-Wei M, Zhang D, Merkle CJ, Ward IM, Saya H, Fang G, van Deursen J, et al. Chfr is required for tumor suppression and Aurora A regulation. Nat Genet. 2005;37:401–406. doi: 10.1038/ng1538. [DOI] [PubMed] [Google Scholar]
- 46.Yang H, He L, Kruk P, Nicosia SV, Cheng JQ. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer. 2006;119:2304–2312. doi: 10.1002/ijc.22154. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Zhou YX, Qiao W, Tominag Y, Ouchi M, Ouchi T, Deng CX. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006;25:7148–7158. doi: 10.1038/sj.onc.1209707. [DOI] [PubMed] [Google Scholar]
- 48.Keen N, Taylor S. Aurora-kinase inhibitors as anticancer agents. Nat Rev Cancer. 2004;4:927–936. doi: 10.1038/nrc1502. [DOI] [PubMed] [Google Scholar]