Abstract
The primary motivation behind the considerable effort in studying stratospheric ozone depletion is the potential for biological consequences of increased solar UVB (280–315 nm) radiation. Yet, direct links between ozone depletion and biological impacts have been established only for organisms of Antarctic waters under the influence of the ozone “hole;” no direct evidence exists that ozone-related variations in UVB affect ecosystems of temperate latitudes. Indeed, calculations based on laboratory studies with plants suggest that the biological impact of ozone depletion (measured by the formation of cyclobutane pyrimidine dimers in DNA) is likely to be less marked than previously thought, because UVA quanta (315–400 nm) may also cause significant damage, and UVA is unaffected by ozone depletion. Herein, we show that the temperate ecosystems of southern South America have been subjected to increasingly high levels of ozone depletion during the last decade. We found that in the spring of 1997, despite frequent cloud cover, the passages of the ozone hole over Tierra del Fuego (55° S) caused concomitant increases in solar UV and that the enhanced ground-level UV led to significant increases in DNA damage in the native plant Gunnera magellanica. The fluctuations in solar UV explained a large proportion of the variation in DNA damage (up to 68%), particularly when the solar UV was weighted for biological effectiveness according to action spectra that assume a sharp decline in quantum efficiency with increasing wavelength from the UVB into the UVA regions of the spectrum.
Keywords: cyclobutane pyrimidine dimer, global change, Gunnera, Tierra del Fuego
The most important consequence of the depletion of stratospheric ozone is the increased transmission of solar UVB radiation to the Earth's surface. Present levels of stratospheric ozone are at the lowest point since measurements began in the 1970s (1). Ozone depletion is most pronounced over the Antarctic continent, where ozone levels commonly decline by more than 70% during late winter and early spring [data available in the NASA Total Ozone Mapping Spectrometer (TOMS) site: http://jwocky.gsfc.nasa.gov/TOMSmain.html]. Acute effects of ozone depletion on native organisms have been documented only for marine ecosystems of Antarctic waters (for a review, see ref. 2). For example, it has been shown that increased UVB can reduce phytoplankton photosynthesis in the marginal ice zone when the ozone hole is overhead (3), reduce phytoplankton cell densities (4), and increase the DNA damage burden in icefish eggs (5). Virtually nothing is known about the consequences of ozone depletion and increased solar UVB on natural ecosystems located outside Antarctica.
We have set up a long-term experiment to study the responses of terrestrial ecosystems to elevated solar UVB resulting from stratospheric ozone reduction near Ushuaia, Tierra del Fuego, Argentina. The area, dominated by temperate forests, is on the southern tip of South America, across the Drake Passage from the Antarctic Peninsula, and experiences more pronounced ozone reduction than any other location in the world where terrestrial ecosystems with appreciable vegetal cover occur. In addition to monitoring long-term responses at the ecosystem level, we wanted to assess the impact of short periods of increased UVB during the passage of the ozone hole on DNA damage density in plants of the local flora. DNA damage was selected as a indicator of acute effects of short exposures to increased UVB because, although short-wave UV radiation can disturb most biological macromolecules, including proteins, lipids, and nucleic acids, studies in animal systems suggest that damage to DNA is the principal cause of cell death and degeneration (5). Cyclobutane pyrimidine dimers (CPD) between adjacent pyrimidine bases make up the major fraction (≈75%) of the aberrant DNA photoproducts induced by UV, and the pyrimidine (6-4)pyrimidinone dimer makes up most of the remaining fraction (6). In plants, both CPD and 6-4 photoproducts have growth-inhibitory effects (7); repair of CPD by a specific photolyase is essential for survival in the presence of UVB radiation (8). UVB effects on DNA can also activate cryptic transposable elements in some species, which might cause mutations beyond the extent of immediate DNA damage (9). Ecophysiological studies have provided indirect, correlative evidence suggesting that the plant growth inhibition caused by high (10) and ambient (11, 12) UVB doses may be related to DNA damage. Our recent work in Tierra del Fuego indicated that current levels of solar UVB in the early spring can inhibit leaf area expansion in the perennial herb Gunnera magellanica (13). Therefore, this species was chosen to study the impacts of ozone depletion on DNA integrity.
Materials and Methods
Study Site and Field Plots.
We selected a field site in the Tierra del Fuego National Park, which is covered by Nothofagus spp. forests and has extensive bogs, scrublands, and heathlands (13–15). In the spring of 1996, we marked 20 permanent plots to study the long-term responses to partial UVB exclusion of a scrub ecosystem near Laguna Negra. The plant community in this system is dominated by Chiliotrichum diffusum (“mata negra”) shrubs and a herbaceous layer composed of a few species of vascular plants. G. magellanica is a perennial herb that spreads by creeping stolons. It is common in the understory of Nothofagus forests and frequently dominates the spaces between shrubs in the scrub communities. Adjacent to the permanent plots, we marked 25 G. magellanica patches to be sampled for UV-induced damage and biochemical responses. These patches were covered with 70- × 70-cm filters that allowed the whole solar UV spectrum to penetrate to the ground (full UV treatment), selectively attenuated the UVB component (−UVB treatment), or blocked most of the UV radiation (no-UV treatment). The filters were installed at the beginning of the growing season (Oct. 6, 1997). The following materials were used: for full-UV treatment, 38-μm thick Aclar plastic film (type 22A, Allied Signal, Pottsville, PA; 10 plots); for −UVB treatment, 100-μm clear polyester plastic (Extra Clear Polyester, JCS Plastic, Lemirada, CA; 10 plots); and for no-UV treatment, 3-mm thick Lexan sheets (Lexan MR5, General Electric), which excluded virtually all radiation below 390 nm (5 plots). The filters in the full-UV and −UVB treatments were perforated to allow rainfall to pass through, avoiding the need of artificial irrigation (15). Measurements of UV penetration through the filters indicated that the slit matrix allowed ≈20% of the plant-weighted (16) solar UV radiation to pass through. Perforation of the Lexan sheets proved impractical; therefore, the no-UV plots, which were used for a period of 15 days only, were watered periodically to match approximately the natural precipitation.
UV and Ozone Data.
UV levels were obtained from the National Science Foundation spectroradiometer located at the Centro Austral de Investigaciones Científicas (Consejo Nacional de Investigaciones Científicas y Téchnicas), in Ushuaia (approximately 20 km to the east of our field site). Ozone data were obtained from the TOMS on board of the Earth Probe NASA satellite (http://jwocky.gsfc.nasa.gov/TOMSmain.html; Overpass Data for 1997) and corroborated for the sampling dates by using data from a Brewer instrument operated by C. Rafanelli and R. Iturraspe at the Centro Austral de Investigaciones Científicas. The spectral UV irradiances obtained from the scanning spectroradiometer were converted into “effective” irradiances by using the following weighting functions: (i) DNA damage normalized at 277 nm (Setlow; ref. 17); (ii) generalized plant damage normalized at 280 nm (Caldwell; ref. 16); (iii) erythema induction normalized at 298 nm (Erythema; ref. 18); (iv) DNA damage in alfalfa seedlings normalized at 300 nm (Quaite; ref. 19); and (v) damage to fish larvae normalized at 283.7 nm (Hunter; refs. 20 and 21) as outlined in ref. 22. Biologically effective UV doses were calculated by integrating the effective irradiances from dawn to midday (13:30 h), and these premidday doses were used as a measure of the UV load received by the plants before sampling. Shorter integration times were not used, because, due to differences in instantaneous cloud cover between Ushuaia (spectroradiometer base) and the National Park (field plots), the correlation between sites is poorer for UV irradiance than for UV doses calculated over multihour periods.
Leaf Sampling and DNA Damage Analysis.
There were 14 sampling dates between Oct. 13, 1997 and Nov. 20, 1997. This time period encompassed a 5-fold variation in biologically effective solar UV doses at ground level (see below) and included three passes of the Antarctic ozone hole [<250 Dobson units (DU)] over our field site. The leaves selected for sampling were those located near the center of the plots and positioned in the upper canopy stratum. At the time of sampling, leaves were typically between one-half and three-quarters of their final size. There were five independent replicates of each UV treatment. In the full-UV and −UVB treatment, each replicate involved two separate plots. At midday (13:30 h), 12 leaves were collected from each of the five independent replicates and placed in dark vials, which were kept on ice during the sampling period (≤ 1.5 h). The vials were then placed in liquid N2 and kept frozen until analysis. The five independent replicates of each UV treatment were kept as independent samples during the laboratory protocols (carried out under orange light), and all the assays were run in duplicate for each sample. DNA was purified from 500 mg of leaf tissue per sample as described in ref. 23 and quantitated with ethidium bromide as outlined in ref. 12. CPD levels were determined by using a sensitive immunoassay (24) with the TDM-2 monoclonal antibody, an alkaline phosphatase-conjugated secondary antibody (Bio-Rad), and a chemiluminescent substrate (CSPD Tropix, Bedford, MA), as indicated in ref. 12. Commercial DNA from herring sperm [10 ng⋅μl−1 in TE buffer (10 mM Tris/1 mM EDTA, pH 8)/10 mM NaCl] was irradiated with known doses of 254-nm UVC in a custom-made irradiator to serve as damage standards in all the blots. To create these standards, the DNA solutions (1 ml) were exposed in flat cuvettes (the optical path through the solution was <1 mm) to obtain a uniform exposure. UVC doses were determined with a calibrated IL-1700 double-monochromator spectroradiometer (International Light, Newburyport, MA).
Results and Discussion
Ozone and UV Variations over Tierra del Fuego.
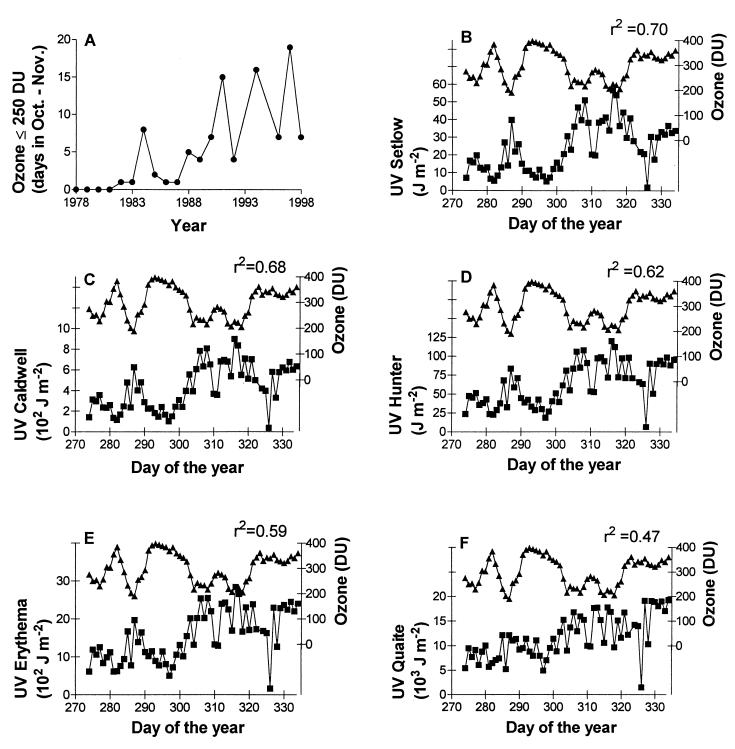
Inspection of the TOMS column ozone data showed that, during the period of 1978–1981, the incipient ozone hole was never large enough to reach the southern tip of South America and that the total ozone values over the region during October and November were consistently above 300 DU. In contrast, after 1982, the ozone hole always passed over Tierra del Fuego in early spring, and the frequency of episodes of very low ozone values (<250 DU) over Ushuaia has increased noticeably during the last 20 years (Fig. 1A). Furthermore, whereas in the early 1980s the hole always vanished before the end of November, in the last several years the ozone hole has persisted until late in the spring. Very recently, on the first week of December 1998, ozone values as low as 200 DU were measured in Ushuaia.
Figure 1.
Ozone depletion and springtime UV levels over Ushuaia. (A) Increase in the number of ozone-hole days over Ushuaia during the past 2 decades. The graph shows the number of days with column ozone values ≤250 DU between Oct. 1 and Nov. 30 for each year. Data were obtained from the Overpass Data file on the internet TOMS site (http://jwocky.gsfc.nasa.gov/TOMSmain.html). No data are available for 1995, and a limited data set is available for October 1993; therefore, these two years are not included in the graph. (B–F) Effects of the passage of the Antarctic ozone hole over Tierra del Fuego on ground-level UV doses during October and November 1997. The spectral irradiances obtained by the National Science Foundation scanning spectroradiometer were converted into effective irradiances by using several weighting functions and integrated over the 9:00–13:30 h time period (■, effective UV dose; ▴, ozone column in Dobson units). The r2 values shown in each panel are from a multiple linear regression model that incorporated Julian day and ozone level as independent variables; in all cases, the ozone term was statistically significant. A comparison of the Erythema-weighted UV doses calculated from spectral irradiance data in Ushuaia with those measured in the field site with a broad-band UV detector (Solar Light, Philadelphia, model PMA2102) indicated a significant linear correlation (r2 = 0.77; 22 data points; November 1997; integration period 9:30–13:30 h).
We found a significant inverse correlation between the variation in column ozone in early spring and the biologically effective UV doses derived from spectral data collected by the National Science Foundation spectroradiometer in Ushuaia (Fig. 1 B–F). As expected, the correlation between ozone content and effective UV was stronger when we used functions to weight the radiation in which the quantum effectiveness declines sharply with the increase in wavelength, because only the shorter wavelengths of the UV spectrum are significantly affected by ozone depletion. Thus, Setlow's action spectrum for DNA damage (17), Caldwell's generalized action spectrum for plant damage (16), and Hunter's spectrum for larval fish growth inhibition (20, 21) gave estimates of effective UV radiation that were strongly correlated with ozone fluctuations during the austral spring. Nonetheless, even the weighting functions that do not strongly discount the UVA region, such as the ones for erythema induction (18) and DNA damage in alfalfa seedlings (19), gave estimates of effective UV doses that were significantly correlated with ozone variations over Ushuaia. These results indicate that, in spite of the large day-to-day variations in cloud cover, the passage of the ozone hole over the southern tip of South America causes large increases in biologically effective UV radiation at the ground level.
UV Fluctuations and DNA Damage.
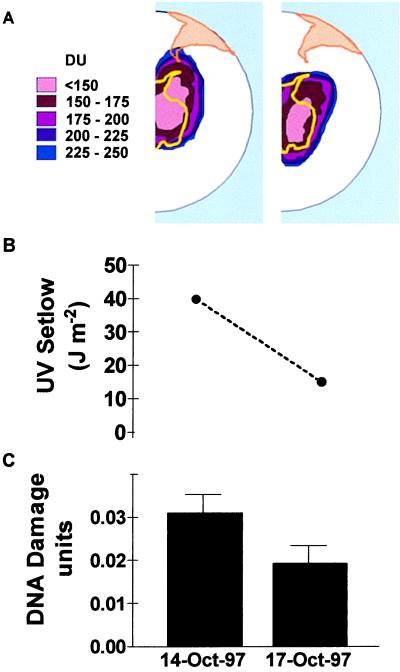
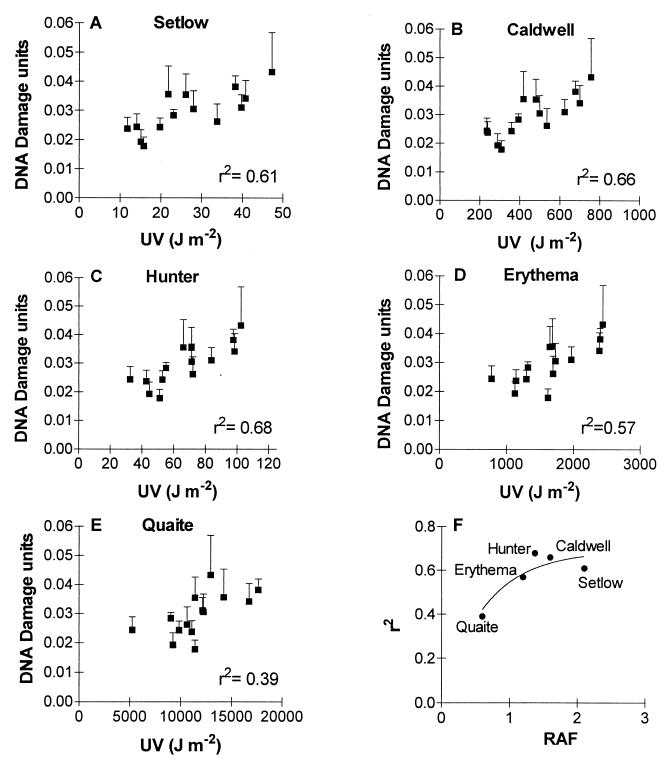
G. magellanica is one of the first species to resume growth at the end of the winter in the southern part of Tierra del Fuego. Our previous work in the National Park shows that, during early spring, G. magellanica leaf area expansion is inhibited by solar UVB (13). Therefore, in the spring of 1997, we collected leaf samples to investigate whether the spikes of solar UV that are commonly observed during ozone-hole days (Fig. 1 B–F) cause increased DNA damage in naturally occurring plants of G. magellanica. In an initial analysis involving five independent replicates, we found that, on Oct. 14, when the ozone hole passed overhead (column ozone = 170 DU), the CPD density in G. magellanica leaves was ≈65% larger than it would be 3 days later, when total ozone had returned to 300 DU and the effective premidday UV dose was ≈2.7 times lower (Fig. 2). Taking into account the results of all 14 sampling dates, we found significant linear relationships between the CPD load at midday and the effective UV dose integrated over the morning hours, although the strength of the relationship depended on the type of action spectrum used to calculate effective UV doses (Fig. 3).
Figure 2.
Ozone depletion and DNA damage. (A) Map of the southern tip of South America and Tierra del Fuego showing the area under the Antarctic ozone hole on Oct. 14 and 17, 1997. (B) Premidday UV doses (weighted by using Setlow's action spectrum). (C) CPD density per nanogram of DNA in leaves of naturally occurring plants of G. magellanica measured at midday (full-UV treatment); 1 unit of DNA damage is defined as the number of CPD produced by 1 J⋅m−2 of 254-nm radiation on 1 ng of purified DNA. Nonirradiated DNA gave no signal in the blots. Each bar is the average of five true replicates.
Figure 3.
CPD density per nanogram of DNA in leaves of G. magellanica plants as a function of the premidday effective UV dose (A–E); 1 unit of DNA damage is defined as the number of CPD produced by 1 J⋅m−2 of 254-nm radiation on 1 ng of purified DNA. Plants are from the full-UV treatment. (F) Relationship between the goodness of fit of the various “DNA damage/UV dose” correlations (A–E) and the radiation amplification factor (RAF) of the weighting function estimated for 30°N latitude, the month of July, and an ozone column of 305 DU (RAF data obtained from ref. 1 and S. Madronich, personal communication).
Wavelength Dependence.
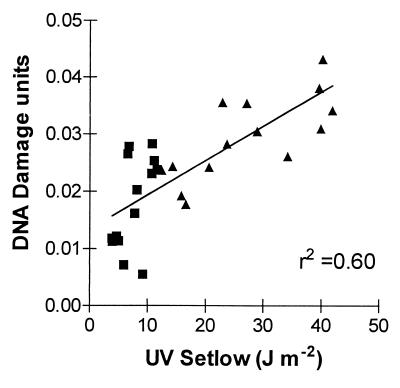
Over the last few years, there has been some debate regarding which wavelengths within the solar UV spectrum cause significant DNA damage under natural conditions (19, 25). Up until now, the only data on wavelength dependence were monochromatic action spectra constructed under laboratory conditions (e.g., refs. 17 and 19). Our field data afford an additional perspective on this issue. The five weighting functions used to estimate effective UV doses from the National Science Foundation radiometric data (Fig. 1) can be compared with regard to their ability to predict the impacts of variations in effective UV on DNA damage. Although the relationship between UV dose and DNA damage was statistically significant for all weighting functions, the steep action spectra (Hunter, Caldwell, and Setlow) tended to give a better prediction of DNA damage density than the action spectra with significant weighting in the UVA region (Fig. 3 A–E). In fact, the percentage of the variance in CPD density that was explained by the different estimates of UV exposure (i.e., the r2 of the CPD/UV relationship shown in Fig. 3 A–E) increased with the RAF of the weighting function used to calculate the biologically effective UV doses [r2 = 0.7(1 − e−1.6*RAF); F = 13.5; P < 0.05; Fig. 3F]. Because the RAF is a measure of the quantum efficiency of the UV wavelengths that are affected by ozone depletion (UVB) relative to those that are not (UVA; ref. 1), these results suggest that most of the variation in DNA damage density in our G. magellanica samples was caused by variations in the UVB component of sunlight. In agreement with this conclusion, the CPD load was consistently higher in the full-UV than in the −UVB plots (repeated-measurement ANOVA; full-UV vs. −UVB treatments; P < 0.01; n = 5; 14 sampling dates), and we failed to detect significant differences in DNA damage density between the −UVB and the no-UV treatment (repeated-measurement ANOVA; −UVB vs. no-UV treatment; P = 0.99; n = 5; five sampling dates; data not shown). Furthermore, the CPD data from the −UVB plots could be accounted for by calculating the effective UV doses under the polyester filters by using Setlow's action spectrum (Fig. 4).
Figure 4.
CPD density per nanogram of DNA in leaves of G. magellanica plants from the full-UV (▴) and −UVB (■) treatments as a function of the effective premidday UV dose; 1 unit of DNA damage is defined as the number of CPD produced by 1 J⋅m−2 of 254-nm radiation on 1 ng of purified DNA. The UV doses were estimated from measurements of the spectral transmittance of the filters and by using Setlow's action spectrum as a weighting function.
Conclusions
Our data indicate that the UV variations that take place during early spring, which to a large extent are caused by ozone depletion (Fig. 1 B–F), result in corresponding changes in DNA damage density in naturally occurring individuals of G. magellanica. A 4-fold increase in Setlow's UV dose (which is the magnitude of the UV change produced by the various passages of the ozone hole over Ushuaia in October and November 1997; Fig. 1B) is predicted to cause, on the average, an ≈2-fold increase in the steady-state level of CPD at midday in G. magellanica (Fig. 3A). These results have points in common with recent observations on UVB-induced DNA damage in icefish eggs in Antarctica (5), although the CPD density/UV correlation seemed to be stronger for G. magellanica than for Antarctic ichthyoplankton. The high correlation in the present case is probably due to the fact that, as a photosynthetic organism, G. magellanica is obligatorily exposed to sunlight, and therefore to solar UV. Animals may afford to colonize habitats less exposed to radiation, and mobile forms may even actively seek shelter in response to high UVB or to environmental variables that correlate with UVB radiation levels (26, 27). The rate of DNA repair should also influence the strength of the correlation between CPD and UVB levels. It is likely that a sizable fraction of the damage detected at midday in G. magellanica is repaired, as it has been shown for maize plants in field experiments (28). However, the linear relationship between CPD density and UV dose (Fig. 3) indicates that the repair rate in G. magellanica is not fast enough to prevent a strong effect of UVB fluctuations on midday CPD levels. Thus, even in the presence of repair mechanisms, the transient accumulation of DNA lesions may be one of the reasons why leaf expansion in G. magellanica is inhibited by ambient UVB in early spring.
At the latitude of Tierra del Fuego the springtime ozone layer under the influence of the Antarctic vortex is up to 50% thinner than in the years before the ozone hole, and the number of days in which the ozone hole reaches the southern tip of South America during the months of active vegetation regrowth (October and November) has increased from 0 in the early 1980s to 10 ± 2 (SEM) in the 1990s (Fig. 1A). To the extent that the observed negative correlation between column ozone and ground-level UV (Fig. 1 B–F) is a general phenomenon (see ref. 29), the linear relationship between UV dose and CPD density that we have detected (Figs. 3 and 4) suggests that springtime ozone depletion has effectively increased the risk of DNA damage for local populations of G. magellanica.
Acknowledgments
We thank Osvaldo Sala and Steve Flint for their contribution to the design and coordination of the project. Special thanks go to Ann Stapleton for her advice on DNA damage analysis and to Toshio Mori for his generous gift of the antibodies used for CPD detection. Carlos Mazza, Oscar Bianciotto, Juan Rosales, and Ricardo Saenz Samaniego helped with sampling and in the maintenance of the permanent field site, and Verónica Herrera and Andrés Arakelian collaborated in the laboratory experiments. This work was supported financially by National Science Foundation Grants IBN 95244 and DEB-9814357 as part of the Terrestrial Ecology and Climate Change Program and also by Agencia Nacional para la Promoción Científica y Tecnológica Grant PICT97 1-00342 and by Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina). We thank the Centro Austral de Investigaciones Científicas for consistent logistic support and the Dirección de Parques Nacionales (Dirección Técnica Regional Patagónica) for their permission to work in the Tierra del Fuego National Park.
Abbreviations
- CPD
cyclobutane pyrimidine dimer
- TOMS
total ozone mapping spectrometer
- DU
Dobson unit
- RAF
radiation amplification factor
References
- 1.Madronich S, McKenzie R L, Björn L O, Caldwell M M. Environmental Effects of Ozone Depletion: 1998 Assessment. Nairobi, Kenya: U. Nations Environ. Programme; 1998. pp. 1–27. [Google Scholar]
- 2.Weiler C S, Penhale P A, editors. Ultraviolet Radiation in Antarctica: Measurements and Biological Effects. Washington DC: Am. Geophys. Union; 1994. [Google Scholar]
- 3.Smith R C, Prézelin B B, Baker K S, Bidigare R R, Boucher N P, Coley T, Karentz D, MacIntyre S, Matlick H A, Menzies D, et al. Science. 1992;255:952–959. doi: 10.1126/science.1546292. [DOI] [PubMed] [Google Scholar]
- 4.Karentz D, Spero H J. J Plankton Res. 1995;17:1771–1789. [Google Scholar]
- 5.Malloy K D, Holman M A, Mitchell D, Detrich H W., III Proc Natl Acad Sci USA. 1997;94:1258–1263. doi: 10.1073/pnas.94.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell D L, Nguyen T D, Cleaver J E. J Biol Chem. 1990;265:5353–5356. [PubMed] [Google Scholar]
- 7.Jiang C-Z, Lee J, Mitchell D L, Britt A B. Proc Natl Acad Sci USA. 1997;94:7441–7445. doi: 10.1073/pnas.94.14.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landry L G, Stapleton A E, Lim J, Hoffman P, Hays J B, Walbot V, Last R L. Proc Natl Acad Sci USA. 1997;94:328–332. doi: 10.1073/pnas.94.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walbot V. Nature (London) 1999;397:398–399. doi: 10.1038/17043. [DOI] [PubMed] [Google Scholar]
- 10.Hidema J, Kumagai T, Sutherland J C, Sutherland B M. Plant Physiol. 1997;113:39–44. doi: 10.1104/pp.113.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballaré C L, Scopel A L, Stapleton A E, Yanovsky M J. Plant Physiol. 1996;112:161–170. doi: 10.1104/pp.112.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazza C A, Battista D, Zima A M, Szwarcberg-Bracchitta M, Giordano C V, Acevedo A, Scopel A L, Ballaré C L. Plant Cell Environ. 1999;22:61–70. [Google Scholar]
- 13.Rousseaux M C, Ballaré C L, Scopel A L, Searles P S, Caldwell M M. Oecologia. 1998;116:528–535. doi: 10.1007/s004420050618. [DOI] [PubMed] [Google Scholar]
- 14.Moore D M. Flora of Tierra del Fuego. Ed. 1. St. Louis: Mo. Bot. Garden; 1983. [Google Scholar]
- 15.Searles P S, Flint S D, Díaz S B, Rousseaux M C, Ballaré C L, Caldwell M M. Global Change Biol. 1999;5:223–228. [Google Scholar]
- 16.Caldwell M M. Photophysiology. 1971;6:131–177. [Google Scholar]
- 17.Setlow R B. Proc Natl Acad Sci USA. 1974;71:3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diffey B L. Photochem Photobiol. 1987;46:55–60. doi: 10.1111/j.1751-1097.1987.tb04735.x. [DOI] [PubMed] [Google Scholar]
- 19.Quaite F E, Sutherland B M, Sutherland J C. Nature (London) 1992;358:576–578. [Google Scholar]
- 20.Hunter J R, Taylor J H, Moser H G. Photochem Photobiol. 1979;29:325–338. doi: 10.1111/j.1751-1097.1979.tb07055.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith R C, Baker K S. In: The Role of Solar Ultraviolet Radiation in Marine Ecosystems. Calkins J, editor. New York: Plenum; 1982. pp. 509–537. [Google Scholar]
- 22.Booth C R, Ehramjian J C, Mestechkina T, Cabasug L W, Robertson J S, Tusson J R, IV. NSF Polar Programs UV Spectroradiometer Network 1995–1997Operations Report. San Diego: Biosph. Instrum.; 1998. [Google Scholar]
- 23.Doyle J J, Doyle J L. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 24.Stapleton A E, Mori T, Walbot V. Plant Mol Biol Rep. 1993;11:230–236. [Google Scholar]
- 25.Quaite F E, Sutherland J C, Sutherland B M. Plant Mol Biol. 1994;24:475–483. doi: 10.1007/BF00024115. [DOI] [PubMed] [Google Scholar]
- 26.Bothwell M L, Sherbot D M J, Pollock C M. Science. 1994;265:97–100. doi: 10.1126/science.265.5168.97. [DOI] [PubMed] [Google Scholar]
- 27.Mazza C M, Zavala J, Scopel A L, Ballaré C L. Proc Natl Acad Sci USA. 1999;96:980–985. doi: 10.1073/pnas.96.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stapleton A E, Thornber C S, Walbot V. Plant Cell Environ. 1997;20:279–290. [Google Scholar]
- 29.McKenzie R, Connor B, Bodeker G. Science. 1999;285:1709–1711. doi: 10.1126/science.285.5434.1709. [DOI] [PubMed] [Google Scholar]