Abstract
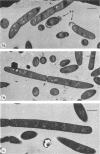
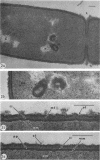
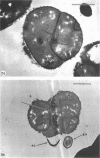
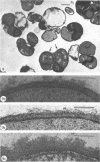
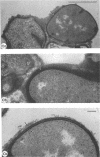
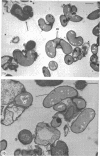
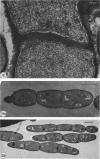
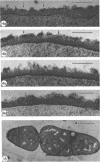
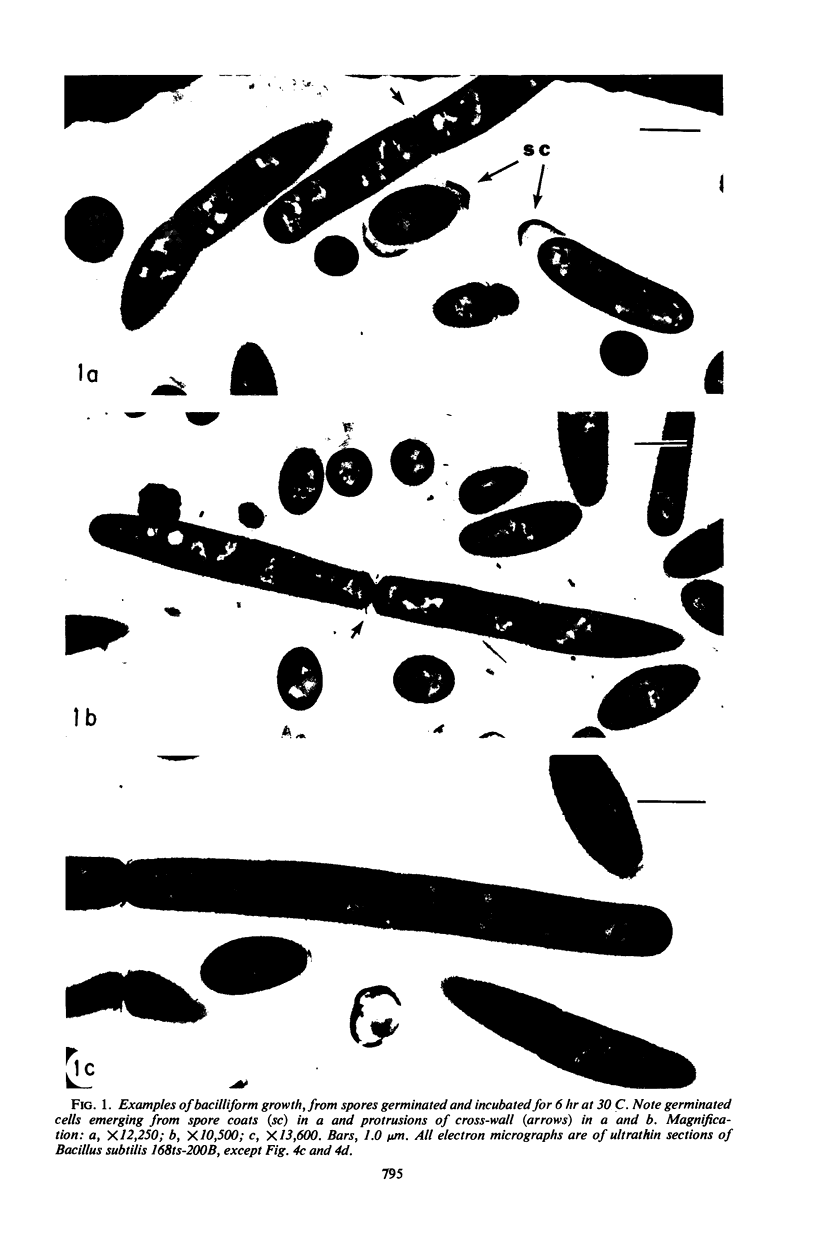
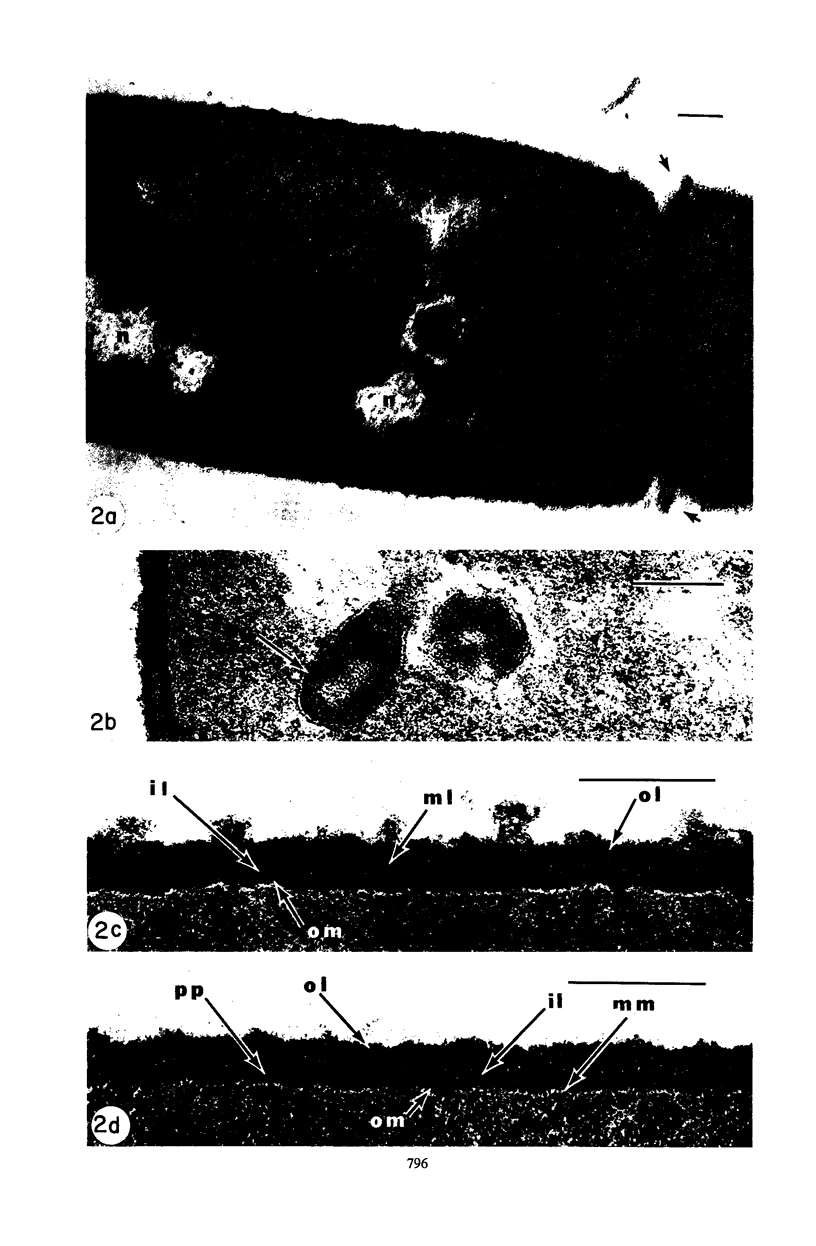
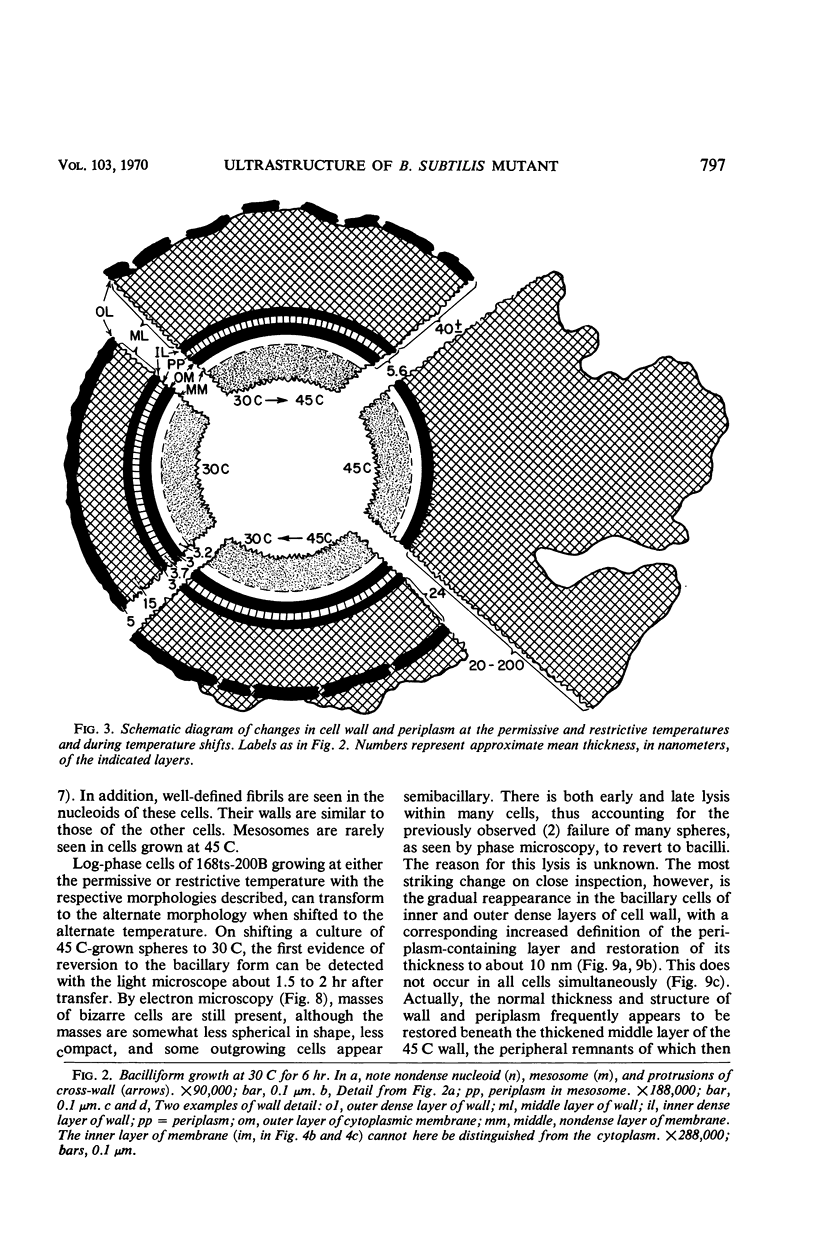
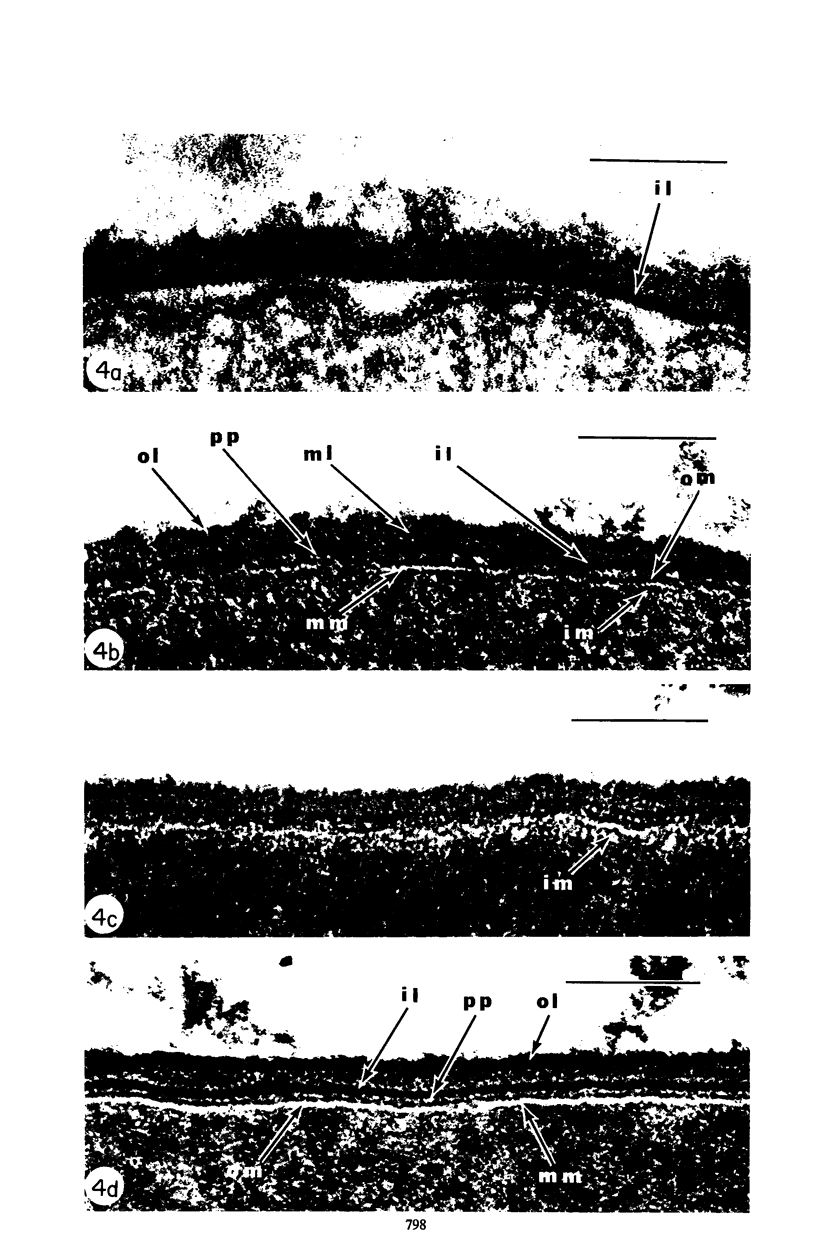
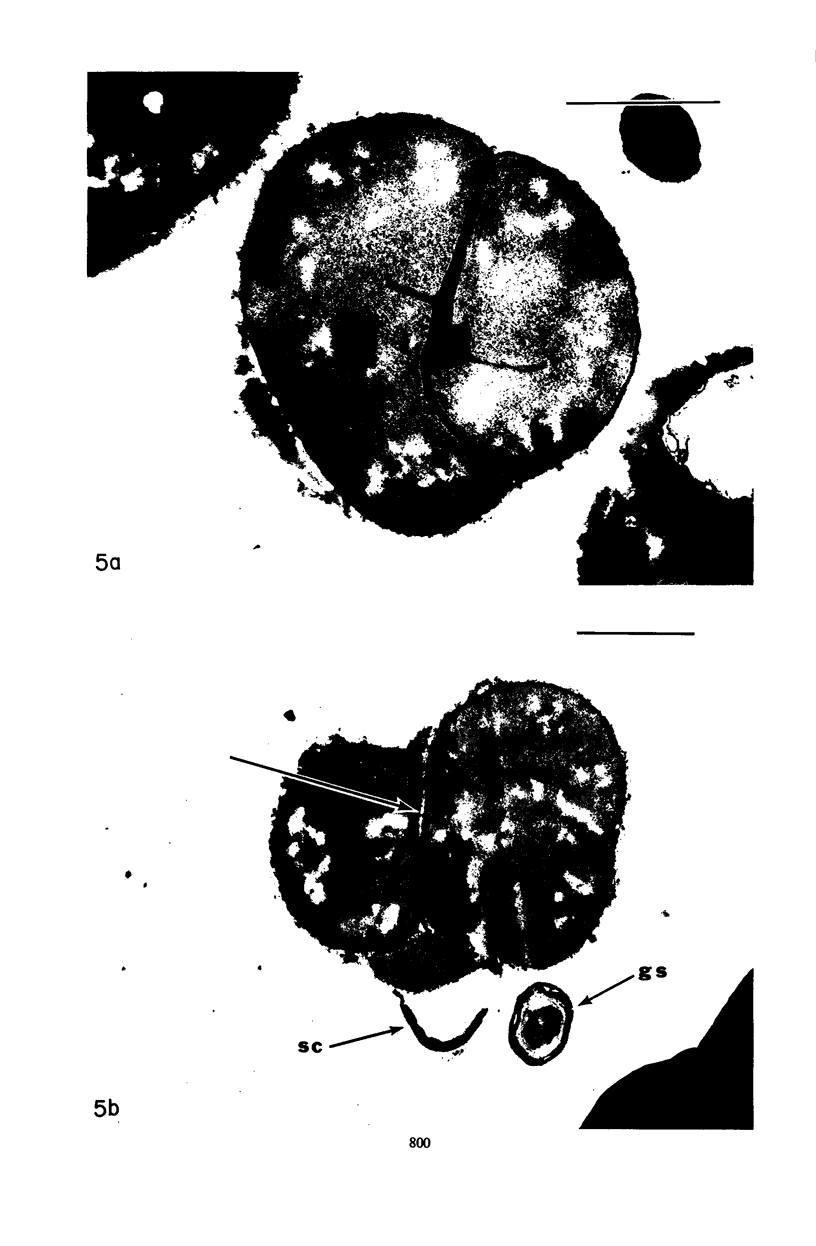
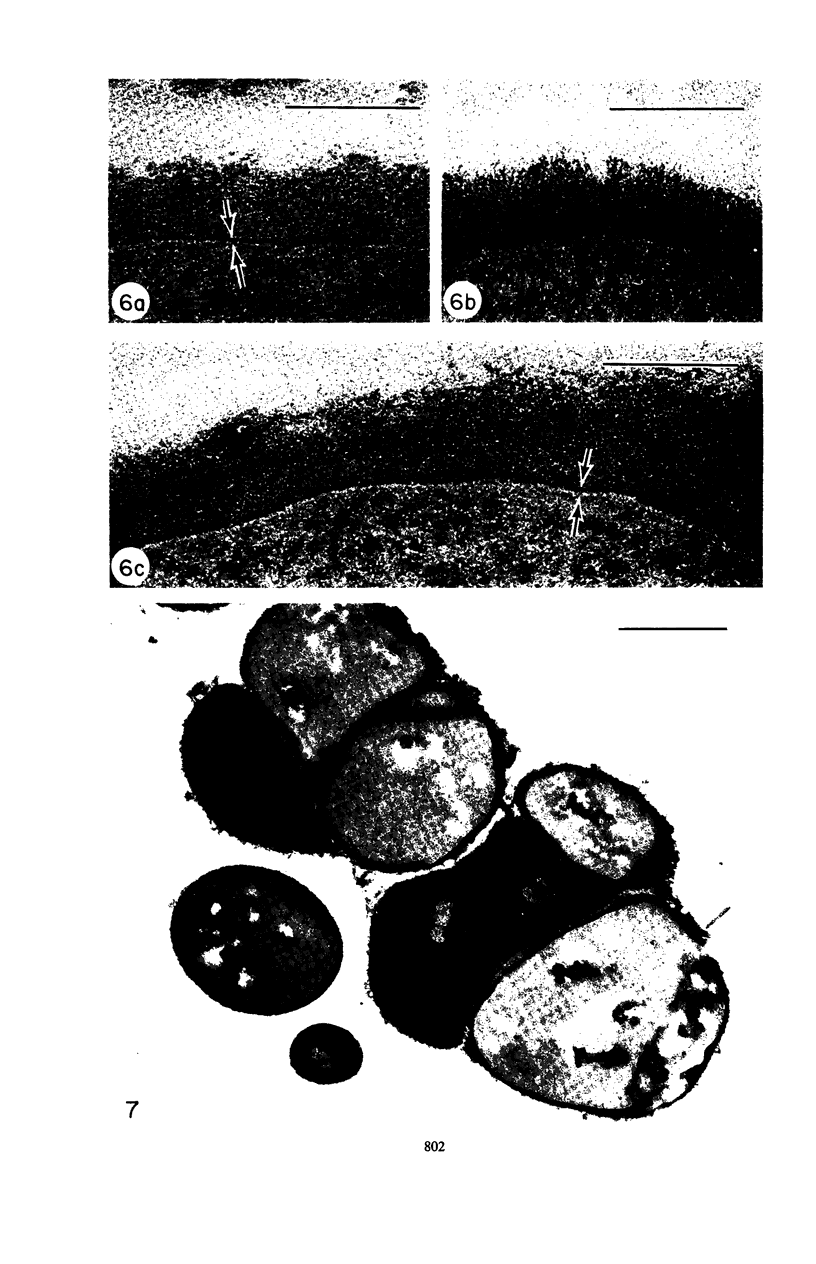
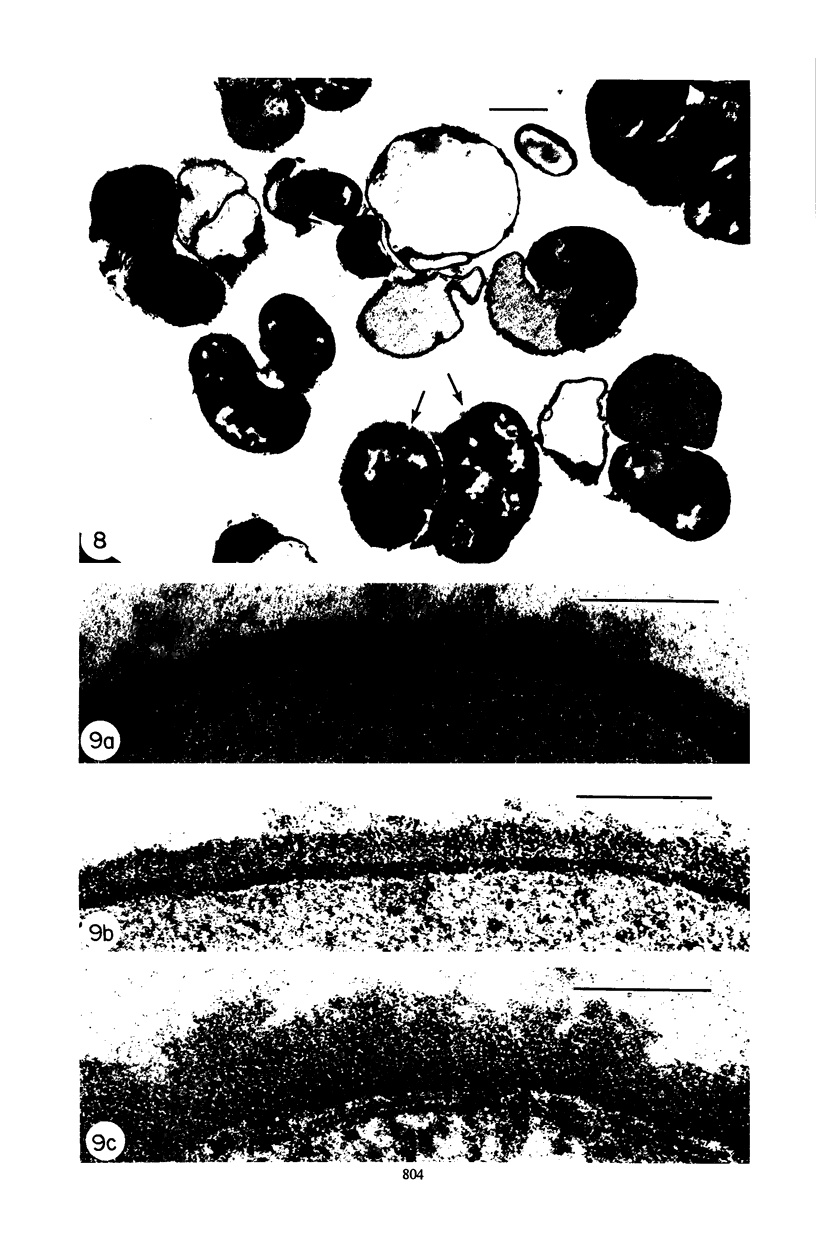
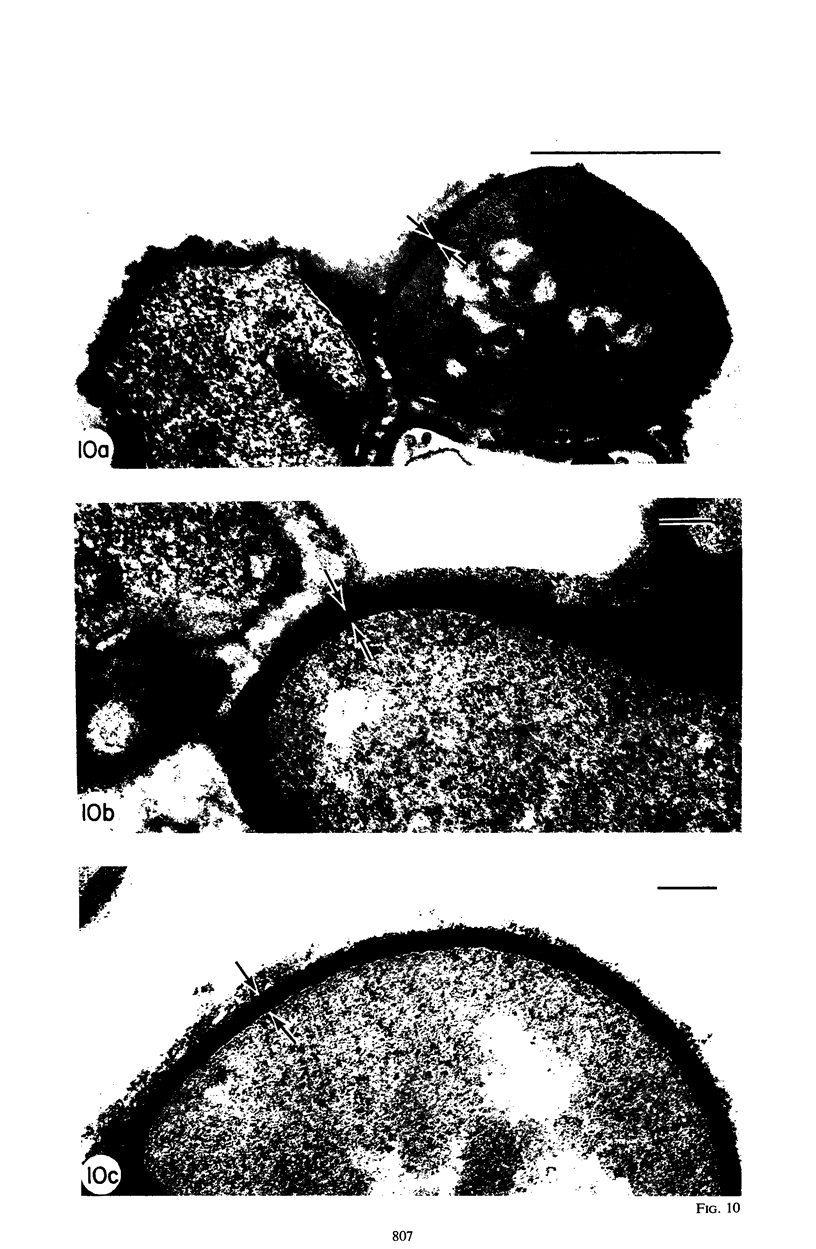
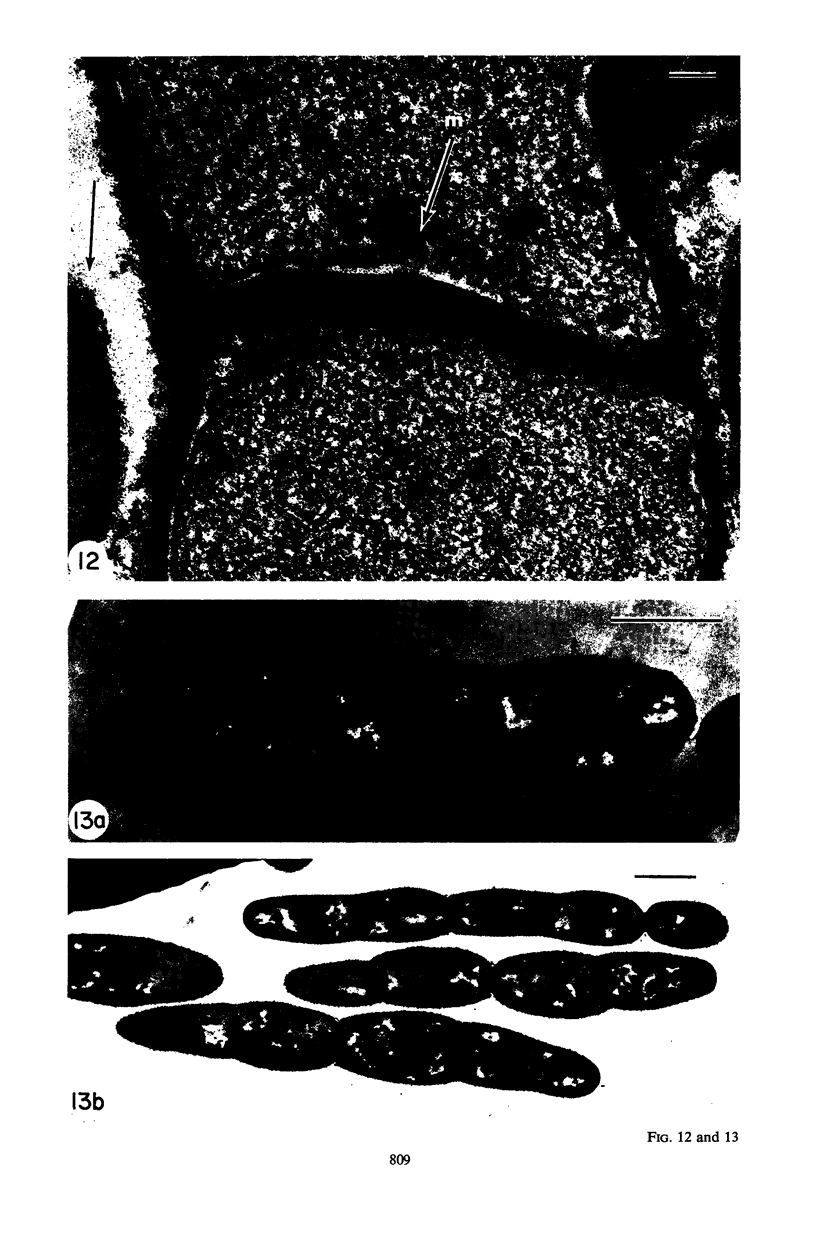
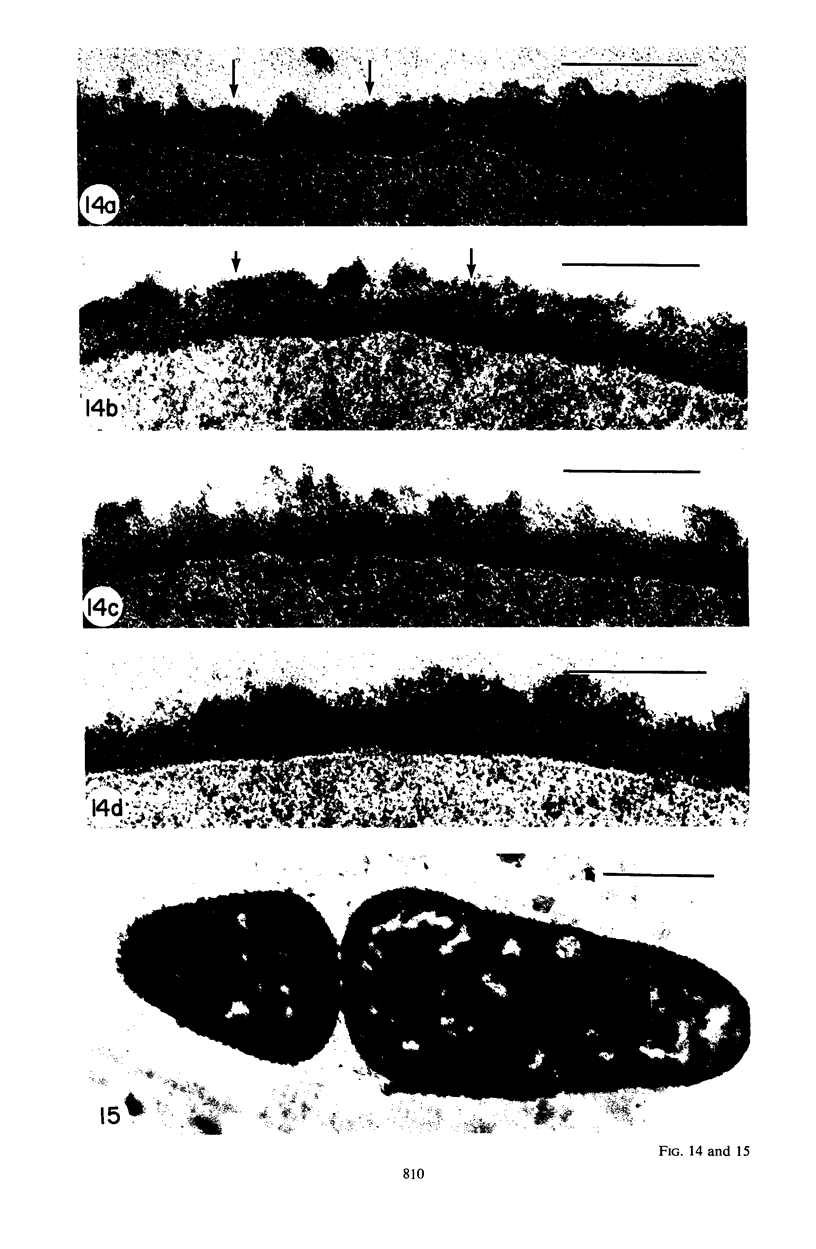
Mutant 168ts-200B, resulting from nitrosoguanidine treatment of Bacillus subtilis 168 (trp− C2), exhibits a rod-to-sphere morphogenetic interconversion when the incubation temperature is 30 or 45 C, respectively. Ultrathin sections of rods grown at 30 C, after glutaraldehyde-osmium uranium-lead fixation and staining, show trilaminar cell walls with a well-developed underlying periplasm as in wild-type cells. However, the outer wall layer is irregular, and abnormal protrusions of wall material occur at the cross-walls. In contrast, cells growing at 45 C become rounded and are intersected randomly by irregular cross-walls which fail to split normally, resulting in large spherical masses. In these, the outer and inner wall layers and periplasm are lost, and the wall consists only of irregularly thickened and loosely organized middle layer. Wall ultrastructure is reversible in either direction as cell shape changes during temperature shifts. Mesosomes are rare and atypical at either temperature. It thus appears that cell wall ultrastructure is altered by the conditional (temperature-sensitive) mutation, and that loss of normal wall and submural organization is correlated with changes in cell size and shape as well as with inability to complete cell division. Preliminary studies after transformation of the mutant locus to another strain and growth at 45 C showed an increase in mucopeptide, loss of wall teichoic acid, failure of phage adsorption, and identical ultrastructural changes. The site of expression of the basic defect—be it in wall, submural region, or membrane—is undetermined.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan R. J., Mendelson N. H. Initial characterization of a temperature-sensitive rod--mutant of Bacillus subtilis. J Bacteriol. 1969 Dec;100(3):1316–1321. doi: 10.1128/jb.100.3.1316-1321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylen C. W., Ensign J. C. Ratio of teichoic acid and peptidoglycan in cell walls of Bacillus subtilis following spire germination and during vegetative growth. J Bacteriol. 1968 Aug;96(2):421–427. doi: 10.1128/jb.96.2.421-427.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Fraser D. K., Young F. E. Problems in purification of a Bacillus subtilis autolytic enzyme caused by association with teichoic acid. Biochim Biophys Acta. 1970 Feb 11;198(2):308–315. doi: 10.1016/0005-2744(70)90063-x. [DOI] [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Mirelman D., Singer H. J., Park J. T. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969 Nov;100(2):846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granboulan P., Leduc E. H. Ultrastructural cytochemistry of Bacillus subtilis. J Ultrastruct Res. 1967 Sep;20(1):111–126. doi: 10.1016/s0022-5320(67)80040-6. [DOI] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Activity of an autolytic N-acetylmuramidase during sphere-rod morphogenesis in Arthrobacter crystallopoietes. J Bacteriol. 1968 Sep;96(3):857–859. doi: 10.1128/jb.96.3.857-859.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. II. Peptides of the cell wall peptidoglycan. J Bacteriol. 1967 Sep;94(3):741–750. doi: 10.1128/jb.94.3.741-750.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty M. The nature of the opaque colony variation in group A streptococci. J Hyg (Lond) 1966 Jun;64(2):185–190. doi: 10.1017/s0022172400040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. Cell wall or membrane mutants of Bacillus subtilis and Bacillus licheniformis with grossly deformed morphology. Nature. 1968 Jul 20;219(5151):285–288. doi: 10.1038/219285a0. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. The organisation of the polymers in Gram-positive and Gram-negative bacteria. J Gen Microbiol. 1969 Aug;57(3):iv–v. [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I. L. The fine structure of Arthrobacter pascens and the development of mesosomes during the growth cycle. Can J Microbiol. 1968 Oct;14(10):1029–1034. doi: 10.1139/m68-173. [DOI] [PubMed] [Google Scholar]
- Swanson J., McCarty M. Electron microscopic studies on opaque colony variants of group A streptococci. J Bacteriol. 1969 Oct;100(1):505–511. doi: 10.1128/jb.100.1.505-511.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren A., Holme T., Häggmark A., Lundquist P. G. Studies on filamentous forms of Bacillus cereus strain T. J Gen Microbiol. 1967 Oct;49(1):59–65. doi: 10.1099/00221287-49-1-59. [DOI] [PubMed] [Google Scholar]
- White D., Dworkin M., Tipper D. J. Peptidoglycan of Myxococcus xanthus: structure and relation to morphogenesis. J Bacteriol. 1968 Jun;95(6):2186–2197. doi: 10.1128/jb.95.6.2186-2197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Fractionation and partial characterization of the products of autolysis of cell walls of Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):839–846. doi: 10.1128/jb.92.4.839-846.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]