Abstract
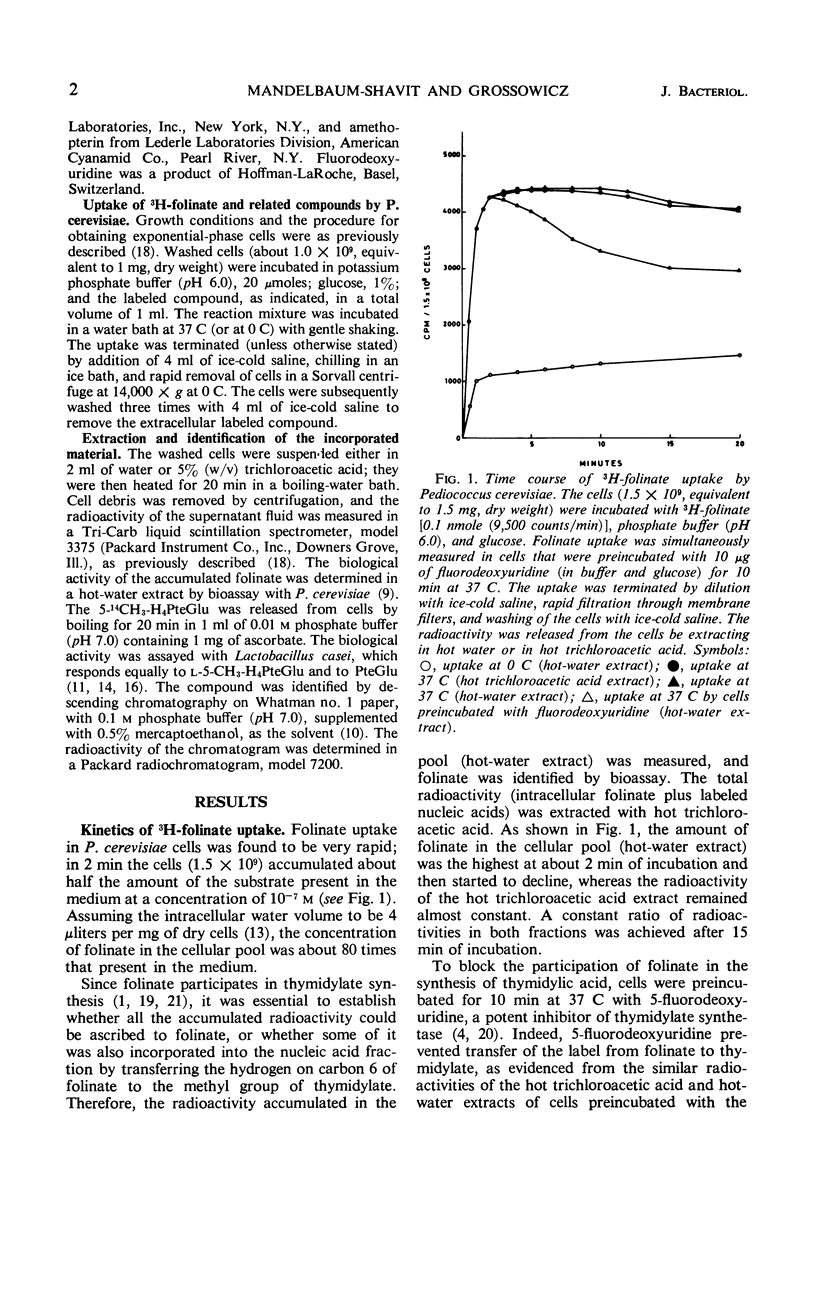
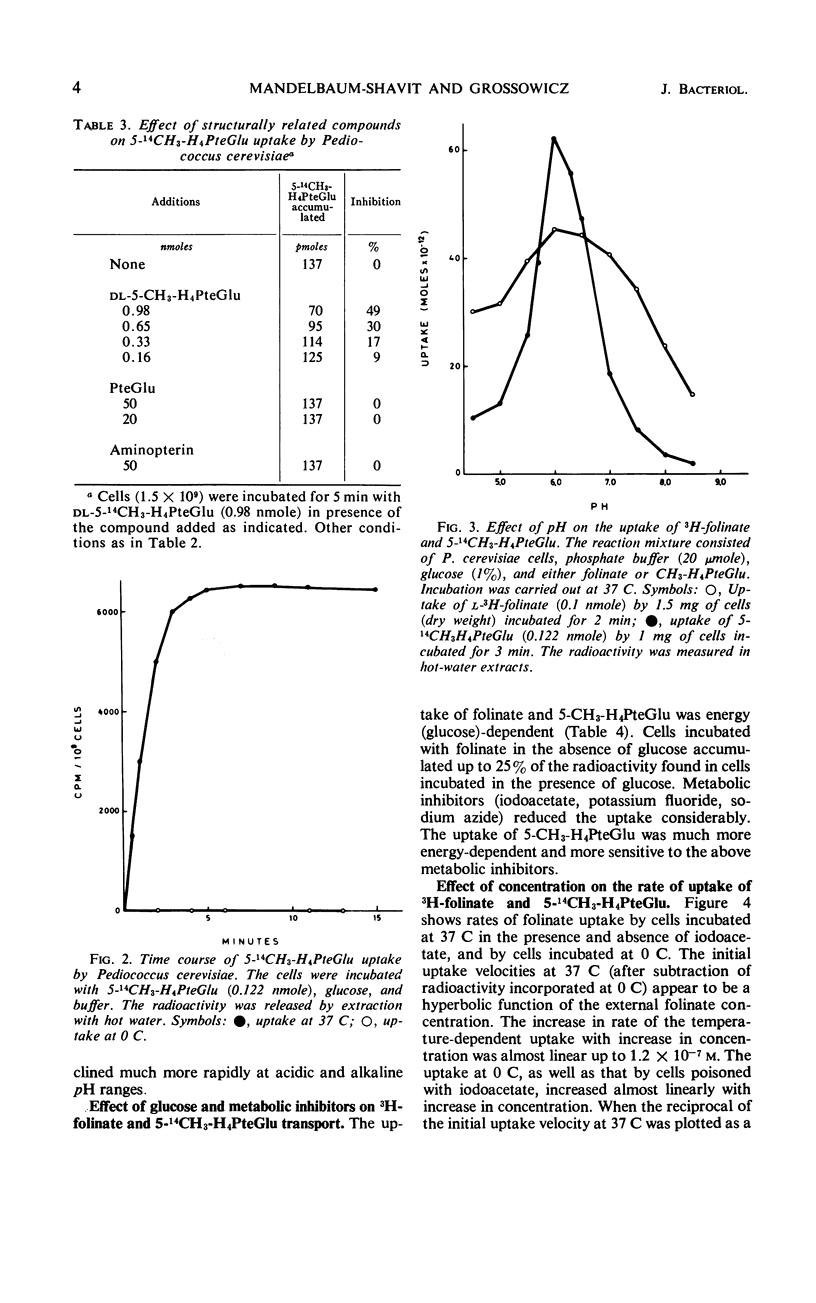
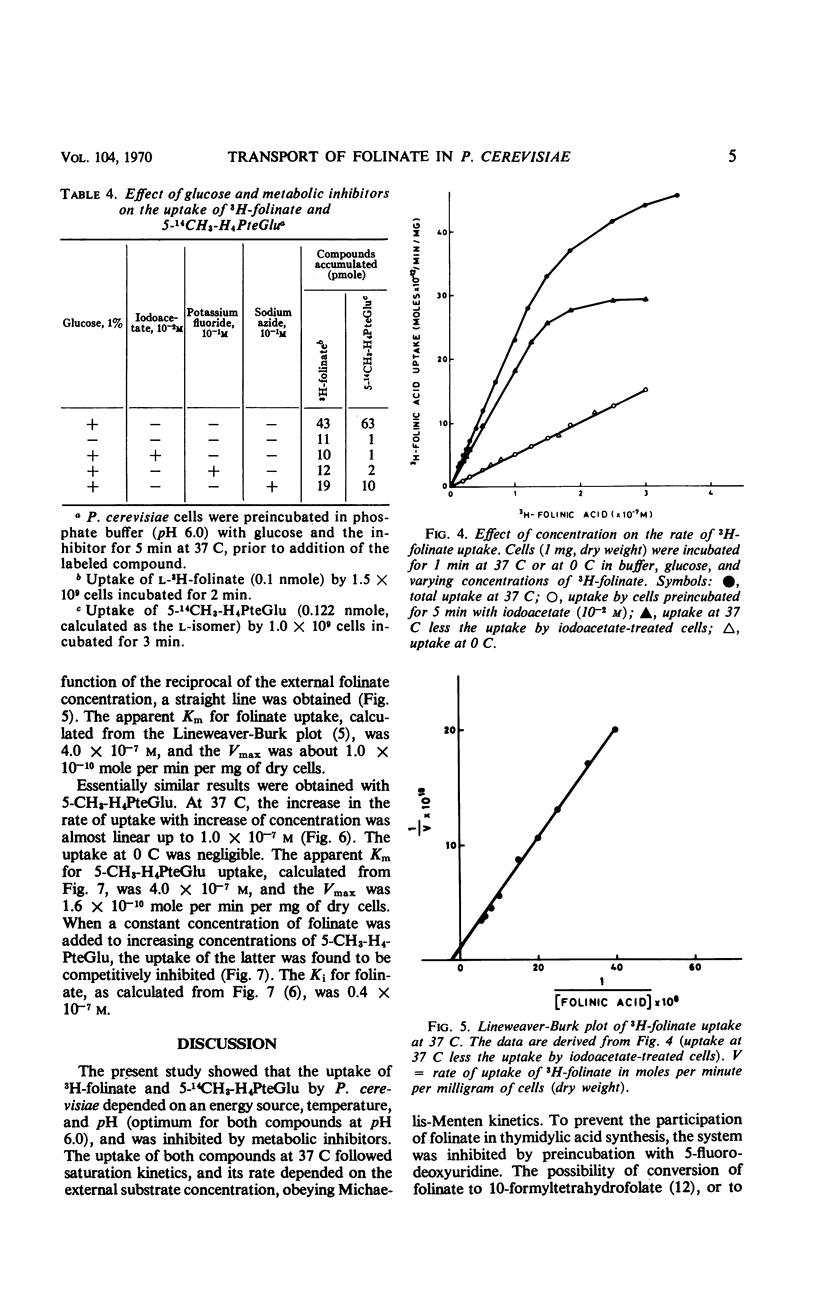
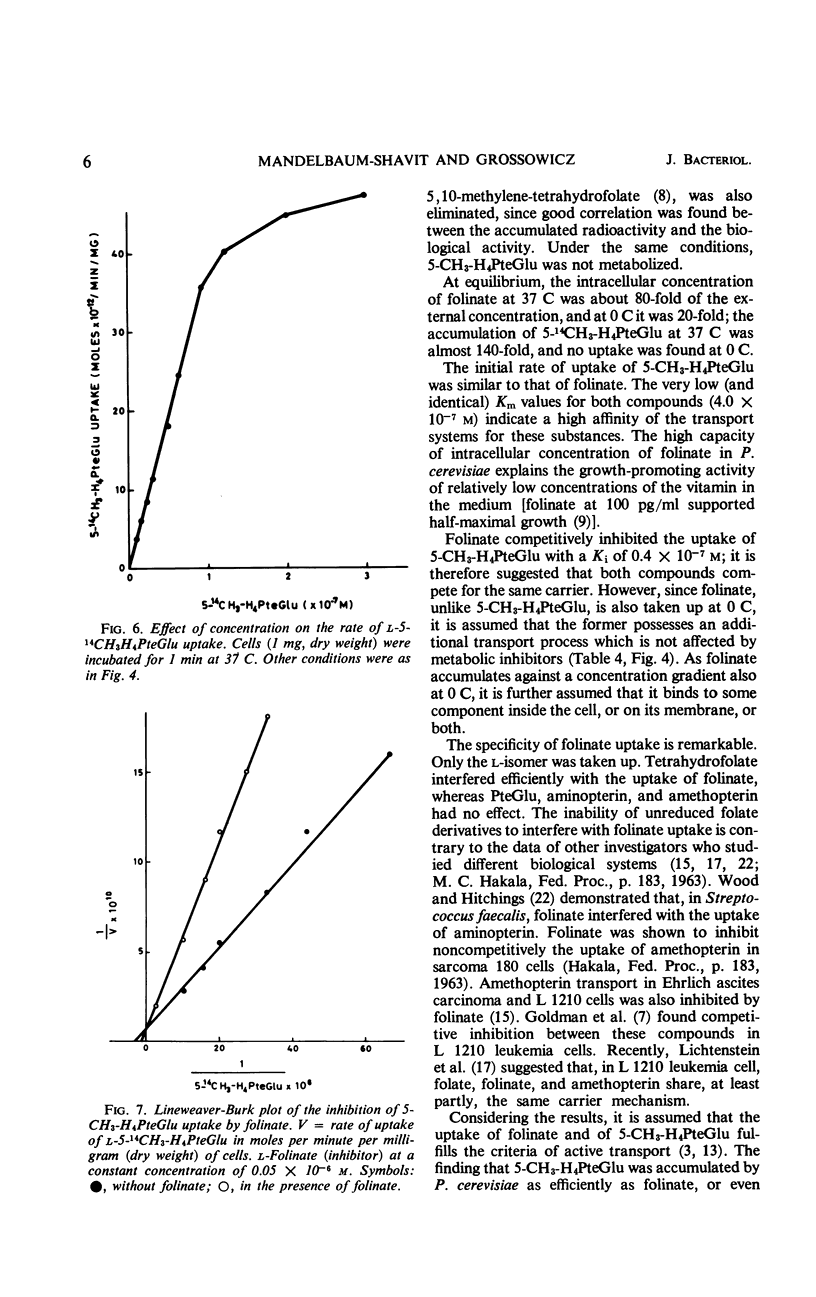
The properties of folinate and 5-methyltetrahydrofolate (5-CH3-H4PteGlu) transport mechanism of Pediococcus cerevisiae were studied. The uptake was dependent on temperature, pH (optimum for both compounds at pH 6.0), and glucose. Iodoacetate, potassium fluoride, and sodium azide inhibited the uptake. 5-CH3-H4-PteGlu was apparently not metabolized but folinate was metabolized. Metabolism of folinate was reduced by preincubation of cells with fluorodeoxyuridine. The transport system for folinate and 5-CH3-H4PteGlu were specific for the l-isomers. Pteroylglutamate, aminopterin, and amethopterin did not interfere with the uptake. Tetrahydrofolate competed with the uptake of folinate. The transport of folinate and 5-CH3-H4PteGlu at 37 C conformed to Michaelis-Menten kinetics; apparent Km for both compounds was 4.0 × 10−7m, and the Vmax for folinate was 1.0 × 10−10 moles per min per mg (dry weight) and for 5-CH3-H4PteGlu it was 1.6 × 10−10 moles per min per mg (dry weight). Both compounds accumulated in the intracellular pool at a concentration about 80- to 140-fold higher than that in the external medium. Folinate inhibited competitively the uptake of 5-CH3-H4PteGlu with a Ki of 0.4 × 10−7m. Unlike 5-CH3-H4PteGlu, which accumulated only at 37 C, folinate was also taken up at 0 C by a glucose- and temperature-independent process, which was not affected by the metabolic inhibitors mentioned above. Since at 0 C the intracellular concentration of folinate was also considerably higher than the external, binding of the substrate to some cellular component is assumed. The finding of an efficient transport system for l-5-CH3-H4PteGlu is of special interest, since this compound has no growth-promoting activity for P. cerevisiae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAKLEY R. L., RAMASASTRI B. V., MCDOUGALL B. M. THE BIOSYNTHESIS OF THYMIDYLIC ACID. V. HYDROGEN ISOTOPE STUDIES WITH DIHYDROFOLIC REDUCTASE AND THYMIDYLATE SYNTHETASE. J Biol Chem. 1963 Sep;238:3075–3079. [PubMed] [Google Scholar]
- Chanarin I., Perry J. A simple method for the preparation of 5-methyltetrahydropteroylglutamic acid. Biochem J. 1967 Nov;105(2):633–634. doi: 10.1042/bj1050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSOWICZ N., MANDELBAUM-SHAVIT F., DAVIDOFF R., ARONOVITCH J. Microbiologic determination of folic acid derivatives in blood. Blood. 1962 Nov;20:609–616. [PubMed] [Google Scholar]
- Goldman I. D., Lichtenstein N. S., Oliverio V. T. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968 Oct 10;243(19):5007–5017. [PubMed] [Google Scholar]
- HERBERT V., LARRABEE A. R., BUCHANAN J. M. Studies on the identification of a folate compound of human serum. J Clin Invest. 1962 May;41:1134–1138. doi: 10.1172/JCI104565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAY L. D., OSBORN M. J., HATEFI Y., HUENNEKENS F. M. The enzymatic conversion of N5-formyl tetrahydrofolic acid (folinic acid) to N10-formyl tetrahydrofolic acid. J Biol Chem. 1960 Jan;235:195–201. [PubMed] [Google Scholar]
- KERESZTESY J. C., DONALDSON K. P. Synthetic prefolic A. Biochem Biophys Res Commun. 1961 Jul 26;5:286–288. doi: 10.1016/0006-291x(61)90164-4. [DOI] [PubMed] [Google Scholar]
- Kessel D., Hall T. C. Amethopterin transport in Ehrlich ascites carcinoma and L1210 cells. Cancer Res. 1967 Sep;27(9):1539–1543. [PubMed] [Google Scholar]
- Lichtenstein N. S., Oliverio V. T., Goldman I. D. Characteristics of folic acid transport in the L1210 leukemia cell. Biochim Biophys Acta. 1969;193(2):456–467. doi: 10.1016/0005-2736(69)90204-1. [DOI] [PubMed] [Google Scholar]
- Mandelbaum-Shavit F., Grossowicz N. Effect of folinate on thymidine uptake by Pediococcus cerevisiae. J Bacteriol. 1968 Apr;95(4):1314–1321. doi: 10.1128/jb.95.4.1314-1321.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTORE E. J., FRIEDKIN M. The enzymatic synthesis of thymidylate. II. Transfer of tritium from tetrahydrofolate to the methyl group of thymidylate. J Biol Chem. 1962 Dec;237:3802–3810. [PubMed] [Google Scholar]
- Reyes P., Heidelberger C. Fluorinated pyrimidines. XXVI. Mammalian thymidylate synthetase: its mechanism of action and inhibition by fluorinated nucleotides. Mol Pharmacol. 1965 Jul;1(1):14–30. [PubMed] [Google Scholar]
- WOOD R. C., HITCHINGS G. H. A study of the uptake and degradation of folic acid, citrovorum factor, aminopterin, and pyrimethamine by bacteria. J Biol Chem. 1959 Sep;234:2381–2385. [PubMed] [Google Scholar]



