Abstract
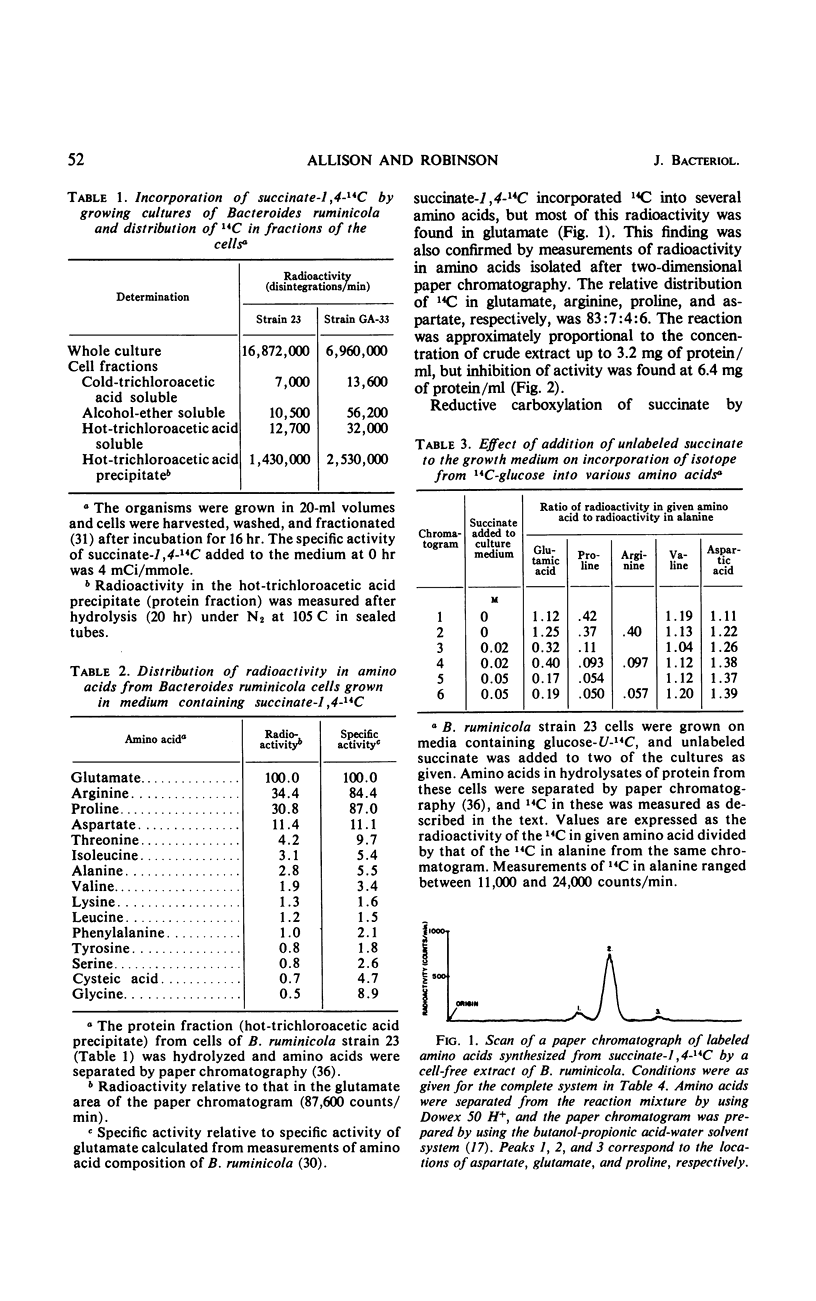
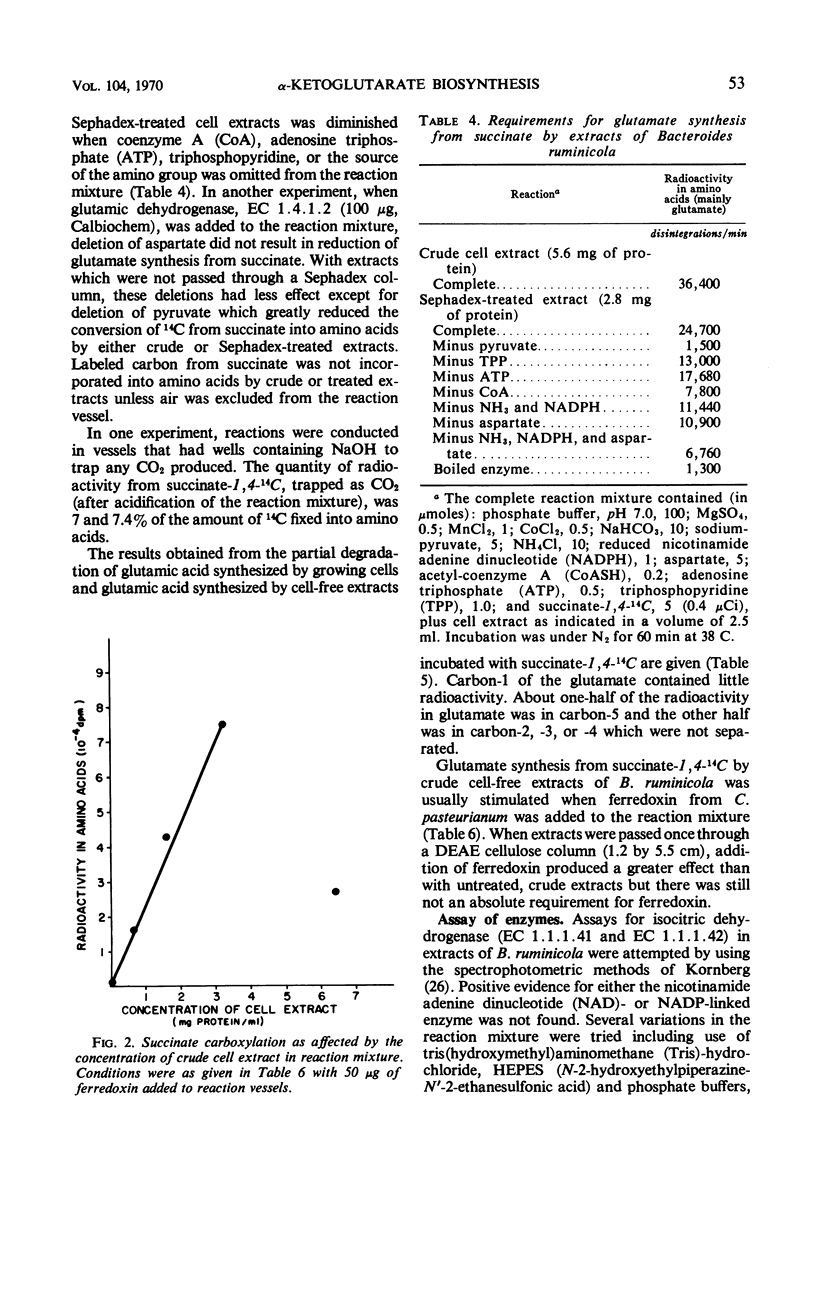
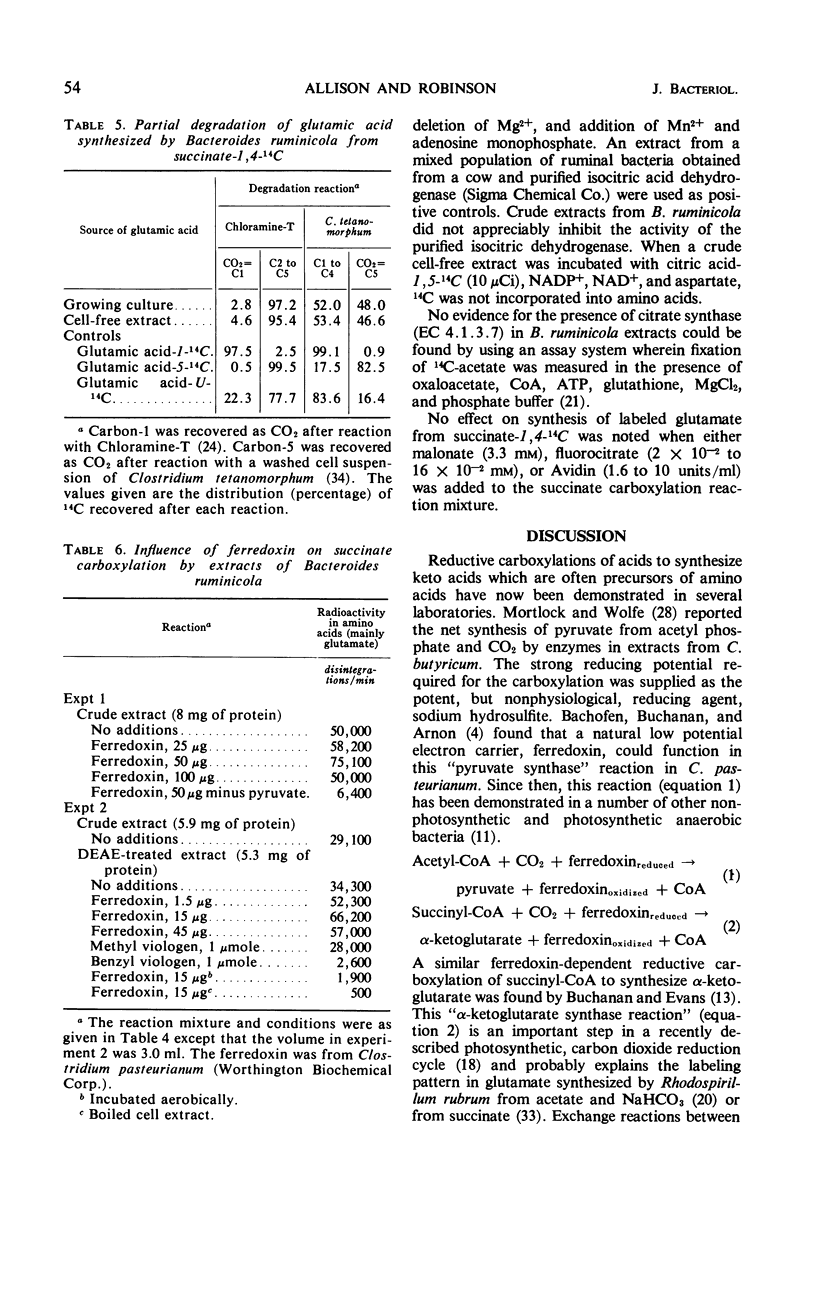
Experiments with growing cells and with cell-free extracts of Bacteroides ruminicola indicate that this anaerobic bacterium can synthesize α-ketoglutarate by a reductive carboxylation of succinate. When the organism was grown in medium containing succinate-1,4-14C, most of the radioactivity in cells was in the protein fraction and most of the 14C in protein was in the glutamic acid family of amino acids (glutamate, proline, and arginine). When unlabeled succinate was added to culture medium containing glucose-U-14C, incorporation of radioactivity into the glutamic acid family of amino acids was greatly reduced. This supports the concept that succinate is an intermediate in synthesis of α-ketoglutarate. Cell-free extracts of the organism incubated with succinate-1,4-14C incorporated 14C into amino acids and most of this was found in glutamate. The cofactors which stimulate glutamate synthesis from succinate by extracts from these cells appear to be similar to the factors that have been demonstrated with extracts from photosynthetic bacteria. The position of label in glutamate synthesized from succinate-1,4-14C, the probable absence of isocitric dehydrogenase, and studies with labeled citrate and with inhibitors of citric acid cycle enzymes support the concept of a reductive carboxylation of succinate as the only, or at least a major, mechanism for synthesis of α-ketoglutarate in this organism. This appears to be the first evidence for a net synthesis of α-ketoglutarate by this reaction in a nonphotosynthetic heterotrophic organism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajl S. J., Werkman C. H. Enzymatic Fixation of Carbon Dioxide in alpha-Ketoglutaric Acid. Proc Natl Acad Sci U S A. 1948 Nov;34(11):491–498. doi: 10.1073/pnas.34.11.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J. Biosynthesis of amono acids by ruminal microorganisms. J Anim Sci. 1969 Nov;29(5):797–807. doi: 10.2527/jas1969.295797x. [DOI] [PubMed] [Google Scholar]
- Allison M. J., Bucklin J. A., Robinson I. M. Importance of the isovalerate carboxylation pathway of leucine biosynthesis in the rumen. Appl Microbiol. 1966 Sep;14(5):807–814. doi: 10.1128/am.14.5.807-814.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHOFEN R., BUCHANAN B. B., ARNON D. I. FERREDOXIN AS A REDUCTANT IN PYRUVATE SYNTHESIS BY A BACTERIAL EXTRACT. Proc Natl Acad Sci U S A. 1964 Apr;51:690–694. doi: 10.1073/pnas.51.4.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHANAN B. B., BACHOFEN R., ARNON D. I. ROLE OF FERREDOXIN IN THE REDUCTIVE ASSIMILATION OF CO2 AND ACETATE BY EXTRACTS OF THE PHOTOSYNTHETIC BACTERIUM, CHROMATIUM. Proc Natl Acad Sci U S A. 1964 Sep;52:839–847. doi: 10.1073/pnas.52.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Evans M. C. The synthesis of alpha-ketoglutarate from succinate and carbon dioxide by a subcellular preparation of a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1212–1218. doi: 10.1073/pnas.54.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B. Role of ferredoxin in the synthesis of alpha-ketobutyrate from propionyl coenzyme A and carbon dioxide by enzymes from photosynthetic and nonphotosynthetic bacteria. J Biol Chem. 1969 Aug 10;244(15):4218–4223. [PubMed] [Google Scholar]
- Cayen M. N., Anastassiadis P. A. A simplified technique for the liquid scintillation measurement of radioactivity on paper chromatograms containing toluene-insoluble C-14-and H-3-labeled compounds. Anal Biochem. 1966 Apr;15(1):84–92. doi: 10.1016/0003-2697(66)90250-8. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY R. W., ALLISON M. J., MULLENAX C. H. PHYSIOLOGICAL DISPOSITION OF C-14-LABELED RUMEN GASES IN SHEEP AND GOATS. Am J Physiol. 1964 Dec;207:1181–1188. doi: 10.1152/ajplegacy.1964.207.6.1181. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B., Arnon D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):928–934. doi: 10.1073/pnas.55.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk G., Barker H. A. Presence and stereospecificity of citrate synthase in anaerobic bacteria. Biochemistry. 1967 Apr;6(4):1027–1034. doi: 10.1021/bi00856a011. [DOI] [PubMed] [Google Scholar]
- HOARE D. S. The photo-assimilation of acetate by Rhodospirillum rubrum. Biochem J. 1963 May;87:284–301. doi: 10.1042/bj0870284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. E., Jr, Baldwin R. L. Enzymatic studies of pure cultures of rumen microorganisms. J Bacteriol. 1966 Nov;92(5):1321–1330. doi: 10.1128/jb.92.5.1321-1330.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMBLE A. R., MACPHERSON H. T. Determination of monoamino monocarboxylic acids by quantitative paper chromatography. Biochem J. 1954 Apr;56(4):548–555. doi: 10.1042/bj0560548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Wolfe R. S., Elsden S. R. The synthesis of amino acids by Methanobacterium omelianskii. Biochem J. 1966 Apr;99(1):76–86. doi: 10.1042/bj0990076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORTLOCK R. P., WOLFE R. S. Reversal of pyruvate oxidation in Clostridium butyricum. J Biol Chem. 1959 Jul;234(7):1657–1658. [PubMed] [Google Scholar]
- PITTMAN K. A., BRYANT M. P. PEPTIDES AND OTHER NITROGEN SOURCES FOR GROWTH OF BACTEROIDES RUMINICOLA. J Bacteriol. 1964 Aug;88:401–410. doi: 10.1128/jb.88.2.401-410.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser D. B., Buechler S. M. Amino acid composition of rumen organisms. J Dairy Sci. 1966 Jan;49(1):81–84. doi: 10.3168/jds.S0022-0302(66)87791-3. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J. Isoleucine biosynthesis from 2-methylbutyric acid by anaerobic bacteria from the rumen. J Bacteriol. 1969 Mar;97(3):1220–1226. doi: 10.1128/jb.97.3.1220-1226.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigesada K., Hidaka K., Katsuki H., Tanaka S. Biosynthesis of glutamate in photosynthetic bacteria. Biochim Biophys Acta. 1966 Jan 4;112(1):182–185. doi: 10.1016/s0926-6585(96)90024-2. [DOI] [PubMed] [Google Scholar]
- WACHSMAN J. T., BARKER H. A. Tracer experiments on glutamate fermentation by Clostridium tetanomorphum. J Biol Chem. 1955 Dec;217(2):695–702. [PubMed] [Google Scholar]
- WHITELEY H. R., McCORMICK N. G. Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J Bacteriol. 1963 Feb;85:382–393. doi: 10.1128/jb.85.2.382-393.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE M. The quantitative determination of amino acids by paper chromatography; a solvent to replace phenol. Biochim Biophys Acta. 1957 Jan;23(1):186–191. doi: 10.1016/0006-3002(57)90302-5. [DOI] [PubMed] [Google Scholar]



