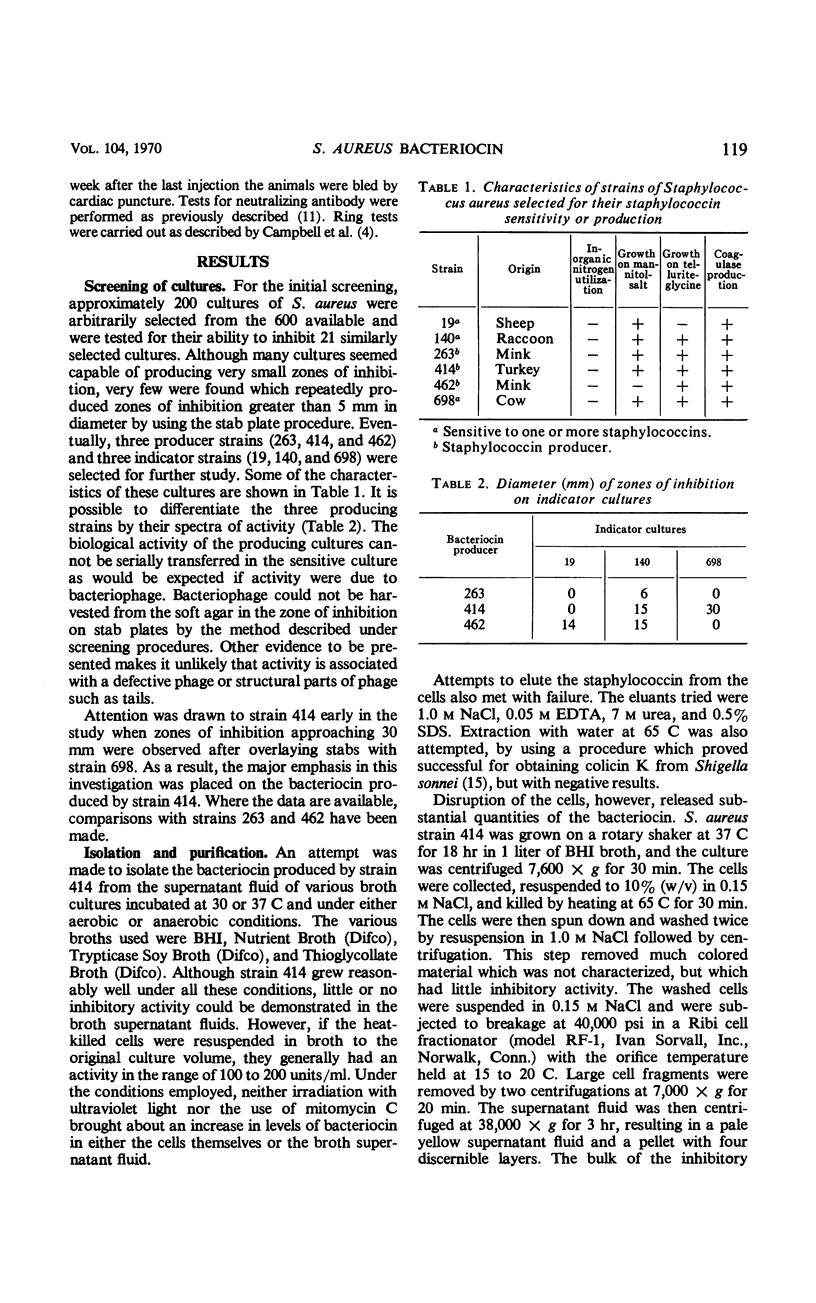
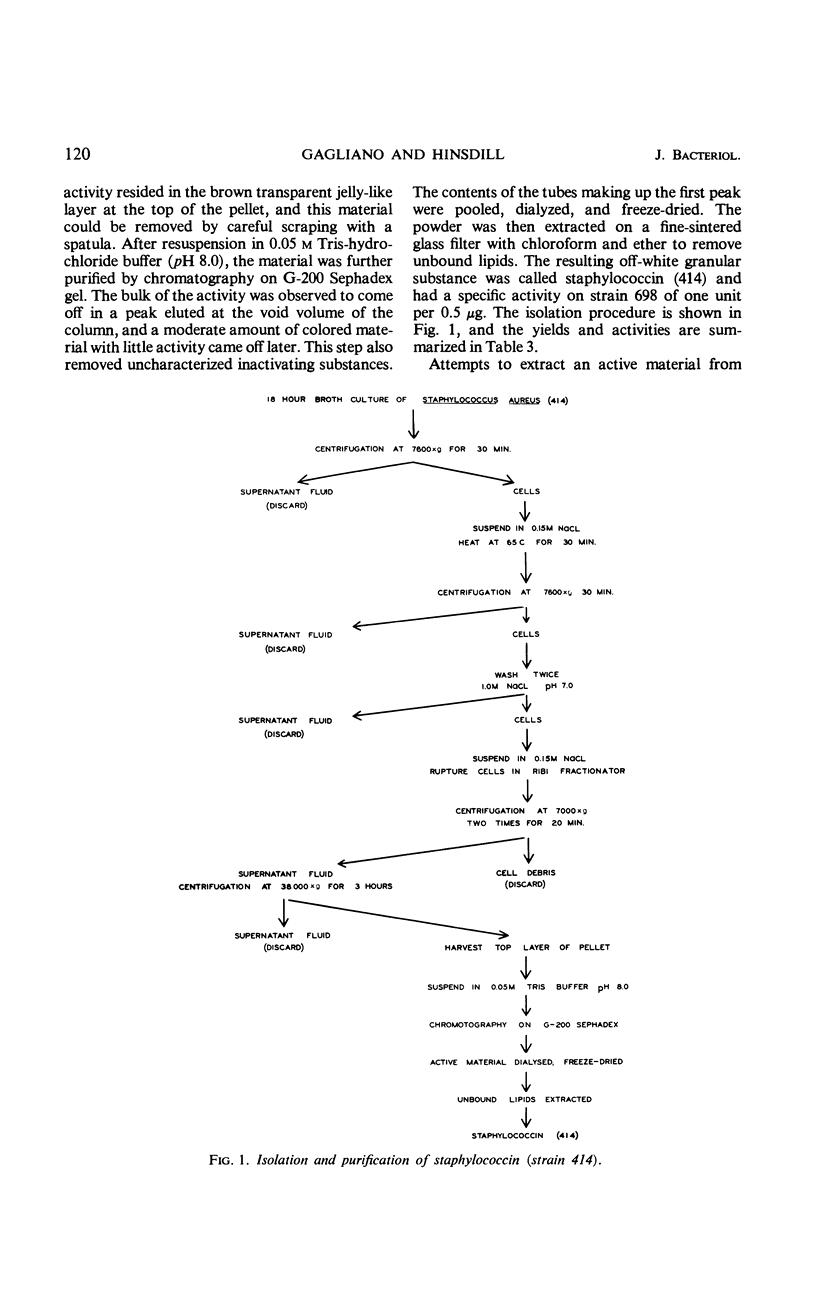
Abstract
The bacteriocin produced by a strain of Staphylococcus aureus has been isolated and designated staphylococcin (414), and a study was made of its chemical, physical, and biological properties. The staphylococcin is released in appreciable quantities after breakage of the cells and can be purified through differential centrifugation and column chromatography. In the native state, it appears to be a lipoprotein-carbohydrate complex with a molecular weight in excess of 200,000. The complex can be dissociated by sodium dodecyl sulfate into smaller subunits which retain activity. The gross chemical and physical properties of the bacteriocin closely resemble those ascribed to certain preparations of cell membranes. Staphylococcin (414) is not a lytic enzyme like lysostaphin and does not have the same spectrum of activity. Like other bacteriocins from gram-positive microorganisms, it does not inhibit any gram-negative bacteria, but does inhibit several other genera.
Full text
PDF

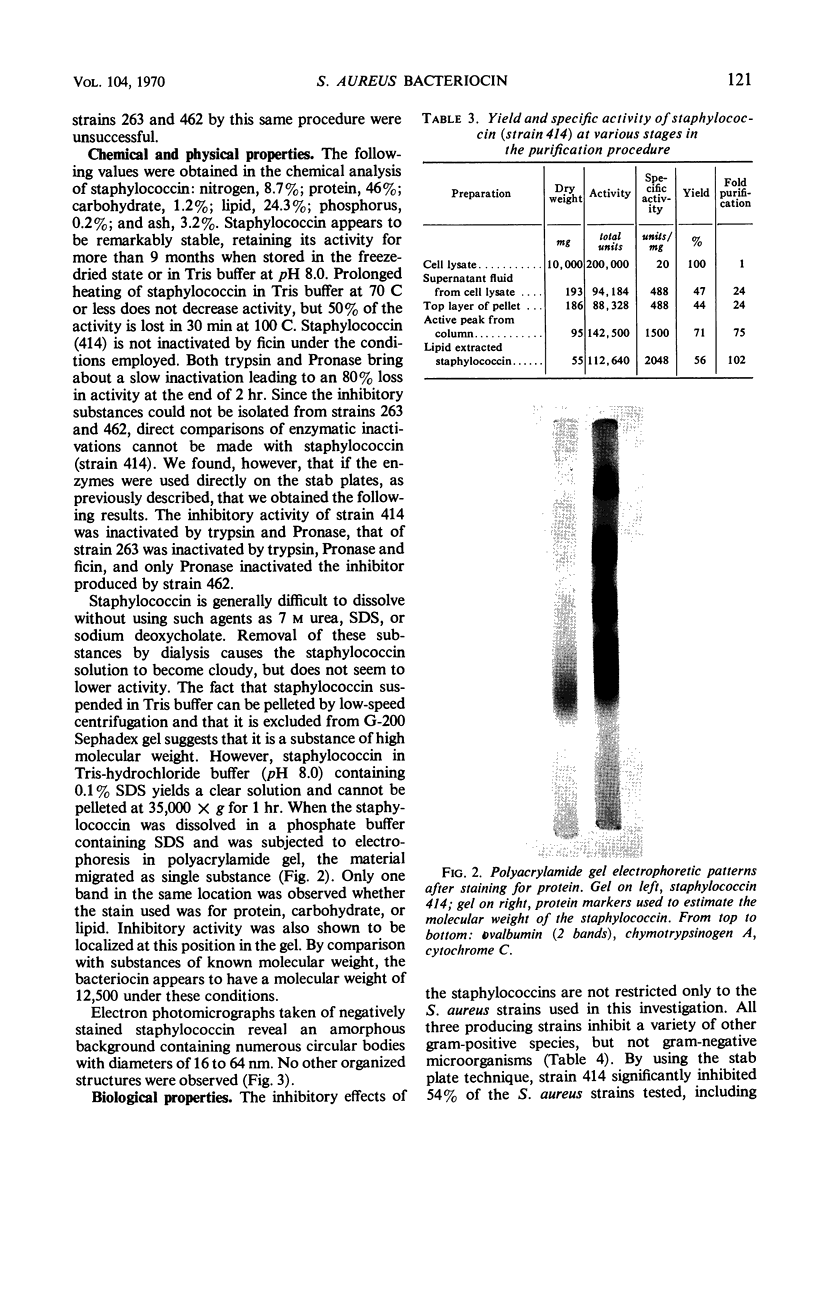
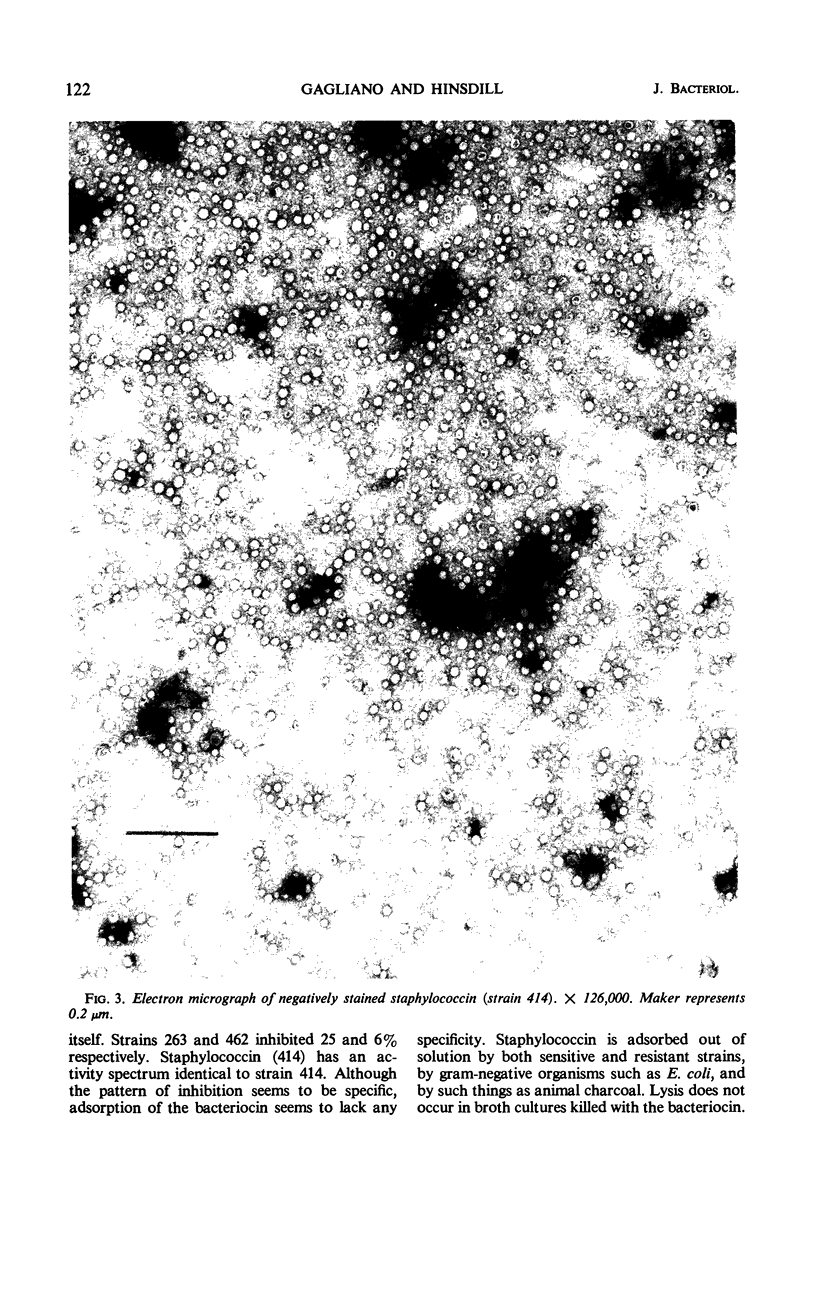



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARROW G. I. THE NATURE OF INHIBITORY ACTIVITY BY STAPHYLOCOCCUS AUREUS TYPE 71. J Gen Microbiol. 1963 Aug;32:255–261. doi: 10.1099/00221287-32-2-255. [DOI] [PubMed] [Google Scholar]
- BROCK T. D., DAVIE J. M. PROBABLE IDENTITY OF A GROUP D HEMOLYSIN WITH A BACTERIOCINE. J Bacteriol. 1963 Oct;86:708–712. doi: 10.1128/jb.86.4.708-712.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- HALBERT S. P., SWICK L., SONN C. Characteristics of antibiotic-producing strains of the ocular bacterial flora. J Immunol. 1953 Apr;70(4):400–410. [PubMed] [Google Scholar]
- HAMON Y., PERON Y. QUELQUES REMARQUES SUR LES BACT'ERIOCINES PRODUITES PAR LES MICROBES GRAM-POSITIFS. C R Hebd Seances Acad Sci. 1963 Jul 29;257:1191–1193. [PubMed] [Google Scholar]
- HUNT G. A., GOUREVITCH A., LEIN J. Preservation of cultures by drying on porcelain beads. J Bacteriol. 1958 Oct;76(4):453–454. doi: 10.1128/jb.76.4.453-454.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsdill R. D., Goebel W. F. Colicine K. VII. The transfer to type K colicinogeny to Shigella sonnei. J Exp Med. 1966 May 1;123(5):881–895. doi: 10.1084/jem.123.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovics G. BACTERIOCINS AND BACTERIOCIN-LIKE SUBSTANCES. Bacteriol Rev. 1962 Jun;26(2 Pt 1):108–118. [PMC free article] [PubMed] [Google Scholar]
- JACOB F. Biosynthèse induite et mode d'action d'une pyocine, antibiotique de Pseudomonas pyocyanea. Ann Inst Pasteur (Paris) 1954 Feb;86(2):149–160. [PubMed] [Google Scholar]
- JACOB F., SIMINOVITCH L., WOLLMAN E. Sur la biosynthèse d'une colicine et sur son mode d'action. Ann Inst Pasteur (Paris) 1952 Sep;83(3):295–315. [PubMed] [Google Scholar]
- KAHN P., HELINSKI D. R. RELATIONSHIP BETWEEN COLICINOGENIC FACTORS E1 AND V AND AN F FACTOR IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1573–1579. doi: 10.1128/jb.88.6.1573-1579.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. The glycerol-phospho-protein complex envelope of Micrococcus pyogenes. J Gen Microbiol. 1951 Nov;5(5 Suppl):981–992. doi: 10.1099/00221287-5-5-981. [DOI] [PubMed] [Google Scholar]
- Maeda A., Nomura M. Interaction of colicins with bacterial cells. I. Studies with radioactive colicins. J Bacteriol. 1966 Feb;91(2):685–694. doi: 10.1128/jb.91.2.685-694.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M. MECHANISM OF ACTION OF COLICINES. Proc Natl Acad Sci U S A. 1964 Dec;52:1514–1521. doi: 10.1073/pnas.52.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER M. T., SIMMONS L. E. The inhibition of Corynebacterium diphtheriae and other gram-positive organisms by Staphylococcus aureus. J Gen Microbiol. 1959 Oct;21:457–476. doi: 10.1099/00221287-21-2-457. [DOI] [PubMed] [Google Scholar]
- RALSTON D. J., LIEBERMAN M., BAER B., KRUEGER A. P. Staphylococcal virolysin, a phage-induced lysin; its differentiation from the autolysis of normal cells. J Gen Physiol. 1957 May 20;40(5):791–807. doi: 10.1085/jgp.40.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUEDE E., GOEBEL W. F. Colicine K. V. The somatic antigen of a non-colicinogenic variant of E. coli K235. J Exp Med. 1962 Jul 1;116:73–100. doi: 10.1084/jem.116.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry T. M., Engelman D. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. II. Modes of aggregation of solubilized membrane components. Biochim Biophys Acta. 1967 Jul 3;135(3):391–405. doi: 10.1016/0005-2736(67)90029-6. [DOI] [PubMed] [Google Scholar]
- Tsao S. S., Goebel W. F. Colicin K. 8. The immunological properties of mitomycin-induced colicin K. J Exp Med. 1969 Dec 1;130(6):1313–1335. doi: 10.1084/jem.130.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]