Abstract
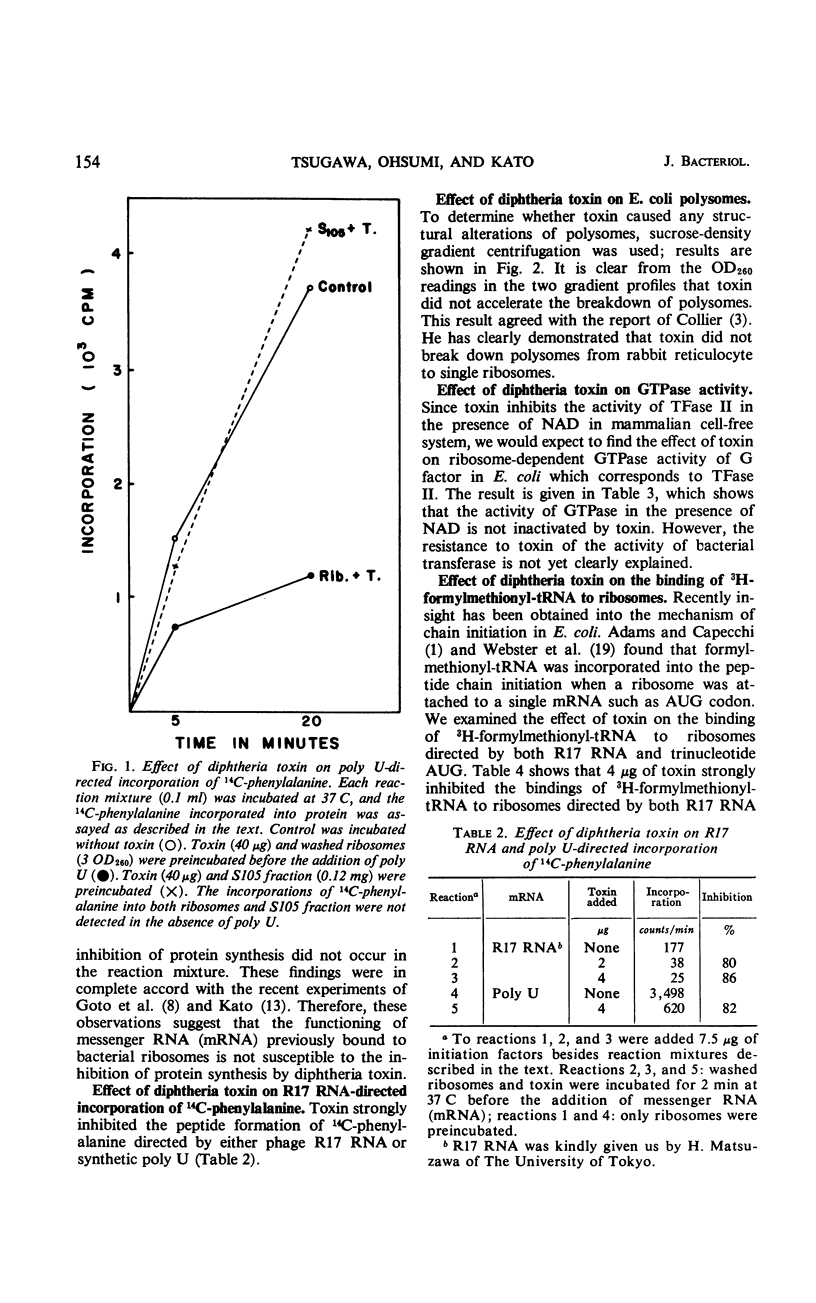
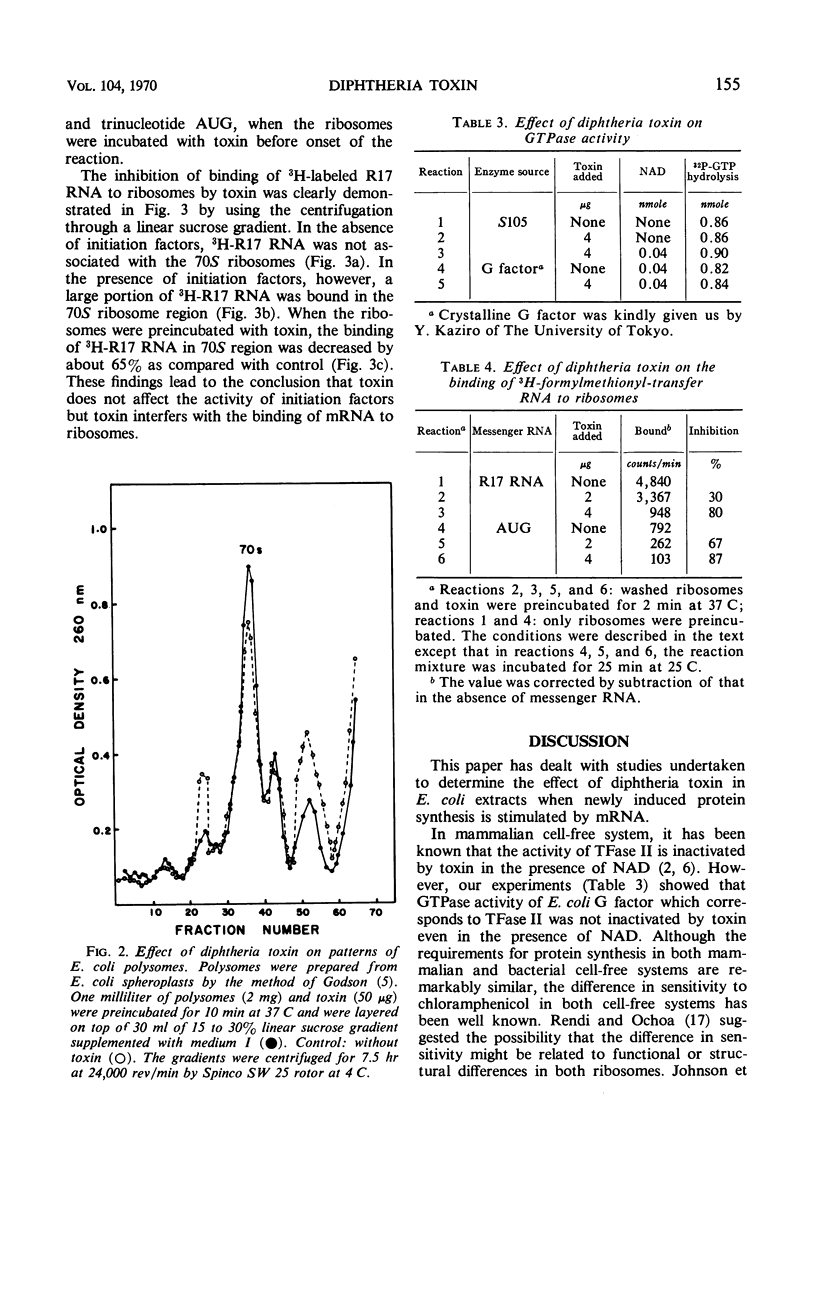
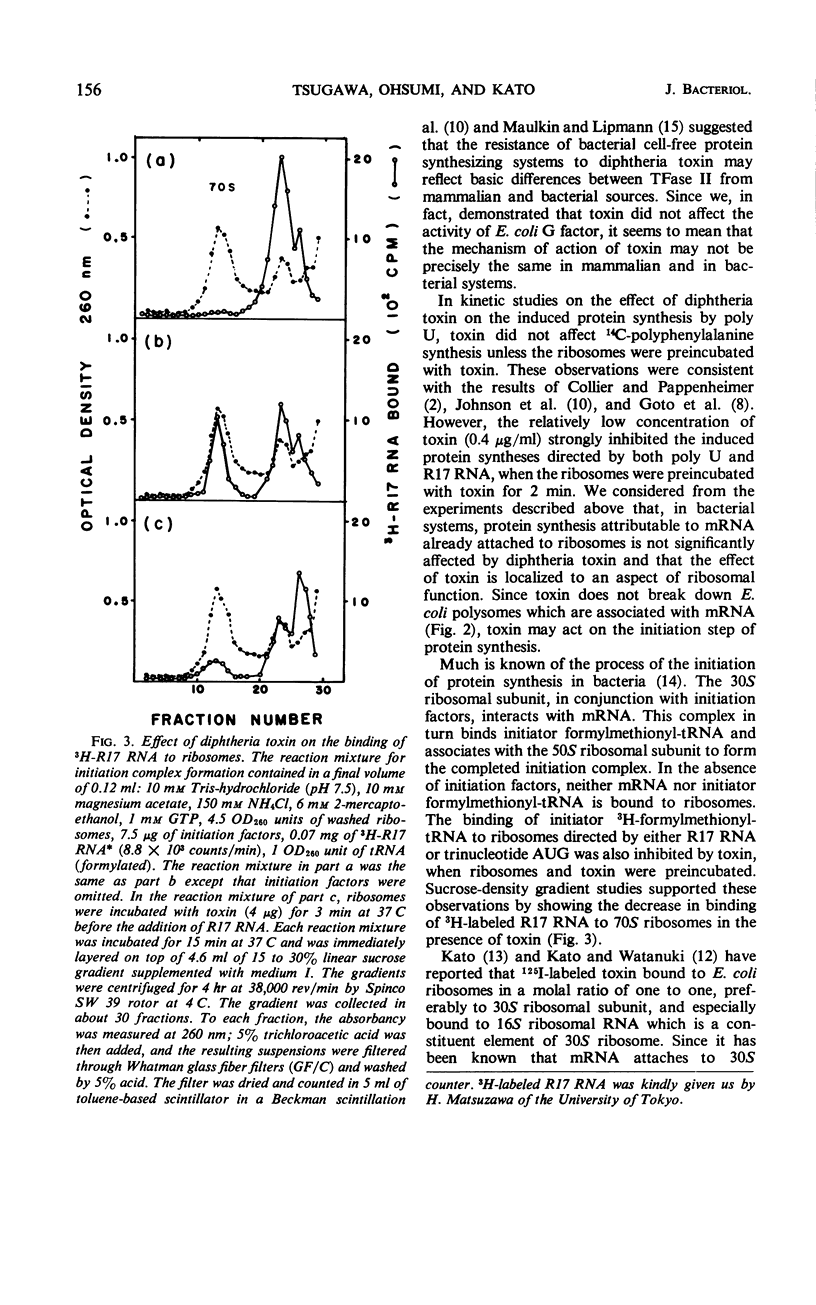
The mechanism of action of diphtheria toxin in an Escherichia coli cell-free protein-synthesizing system was examined. When the washed ribosomes were incubated with toxin before addition of messenger ribonucleic acid (RNA), peptide syntheses of 14C-phenylalanine directed by polyuridylic acid or phage R17 RNA were strongly inhibited by a small amount of toxin. Whereas, if the soluble fraction (105,000 × g supernatant fraction) was preincubated with toxin, no effect of toxin occurred either on the induced protein synthesis or on the activity of guanosine triphosphatase even in the presence of nicotinamide adenine dinucleotide. The binding of 3H-formylmethionyl-transfer RNA to E. coli ribosomes directed by either R17 RNA or trinucleotide AUG was also decreased by toxin. These findings suggest that diphtheria toxin may prevent the binding of messenger RNA by successfully competing with the AUG for ribosomal binding sites. Sucrose-density gradient studies support this concept by showing the decrease in binding of 3H-labeled R17 RNA to E. coli ribosomes exposed to toxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIER R. J., PAPPENHEIMER A. M., Jr STUDIES ON THE MODE OF ACTION OF DIPHTHERIA TOXIN. II. EFFECT OF TOXIN ON AMINO ACID INCORPORATION IN CELL-FREE SYSTEMS. J Exp Med. 1964 Dec 1;120:1019–1039. doi: 10.1084/jem.120.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY T. W., LIPMANN F. CHARACTERIZATION OF A RIBOSOME-LINKED GUANOSINE TRIPHOSPHATASE IN ESCHERICHIA COLI EXTRACTS. Proc Natl Acad Sci U S A. 1964 Dec;52:1462–1469. doi: 10.1073/pnas.52.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Goor R. S., Pappenheimer A. M., Jr, Ames E. Studies on the mode of action of diphtheria toxin. V. Inhibition of peptide bond formation by toxin and NAD in cell-free systems and its reversal by nicotinamide. J Exp Med. 1967 Nov 1;126(5):923–939. doi: 10.1084/jem.126.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goor R. S., Pappenheimer A. M., Jr Studies on the mode of action of diphtheria toxin. 3. Site of toxin action in cell-free extracts. J Exp Med. 1967 Nov 1;126(5):899–912. doi: 10.1084/jem.126.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N., Kato I., Sato H. The inhibitory effect of diphtheria toxin on amino acid incorporation by a bacterial cell-free system. Jpn J Exp Med. 1968 Jun;38(3):185–192. [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- Johnson W., Kuchler R. J., Solotorovsky M. Site in cell-free protein synthesis sensitive to diphtheria toxin. J Bacteriol. 1968 Oct;96(4):1089–1098. doi: 10.1128/jb.96.4.1089-1098.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO I., NAKAMURA H., UCHIDA T., KOYAMA J., KATSURA T. Purification of diphtheria toxin. II. The isolation of crystalline toxin-protein and some of its properties. Jpn J Exp Med. 1960 Apr;30:129–145. [PubMed] [Google Scholar]
- Lengyel P., Söll D. Mechanism of protein biosynthesis. Bacteriol Rev. 1969 Jun;33(2):264–301. doi: 10.1128/br.33.2.264-301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin M., Lipmann F. Fusidic acid: inhibition of factor T2 in reticulocyte protein synthesis. Science. 1969 Apr 4;164(3875):71–72. doi: 10.1126/science.164.3875.71. [DOI] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Response of cultured mammalian cells to diphtheria toxin. 3. Inhibition of protein synthesis studied at the subcellular level. J Bacteriol. 1968 Jul;96(1):61–69. doi: 10.1128/jb.96.1.61-69.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENDI R., OCHOA S. Effect of chloramphenicol on protein synthesis in cell-free preparations of Escherichia coli. J Biol Chem. 1962 Dec;237:3711–3713. [PubMed] [Google Scholar]
- VON EHRENSTEIN G., LIPMANN F. Experiments on hemoglobin biosynthesis. Proc Natl Acad Sci U S A. 1961 Jul 15;47:941–950. doi: 10.1073/pnas.47.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D. In vitro protein synthesis: chain initiation. Proc Natl Acad Sci U S A. 1966 Jan;55(1):155–161. doi: 10.1073/pnas.55.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]



