Abstract
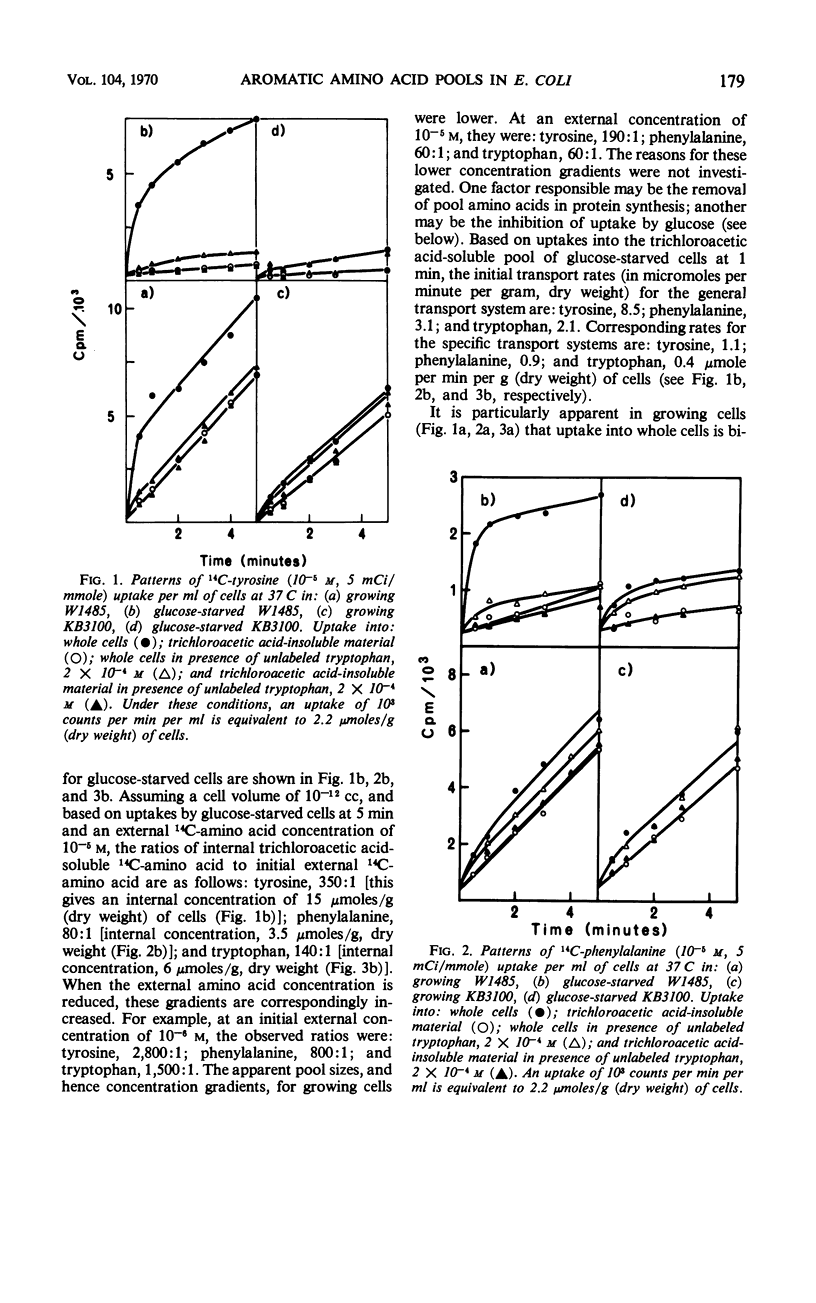
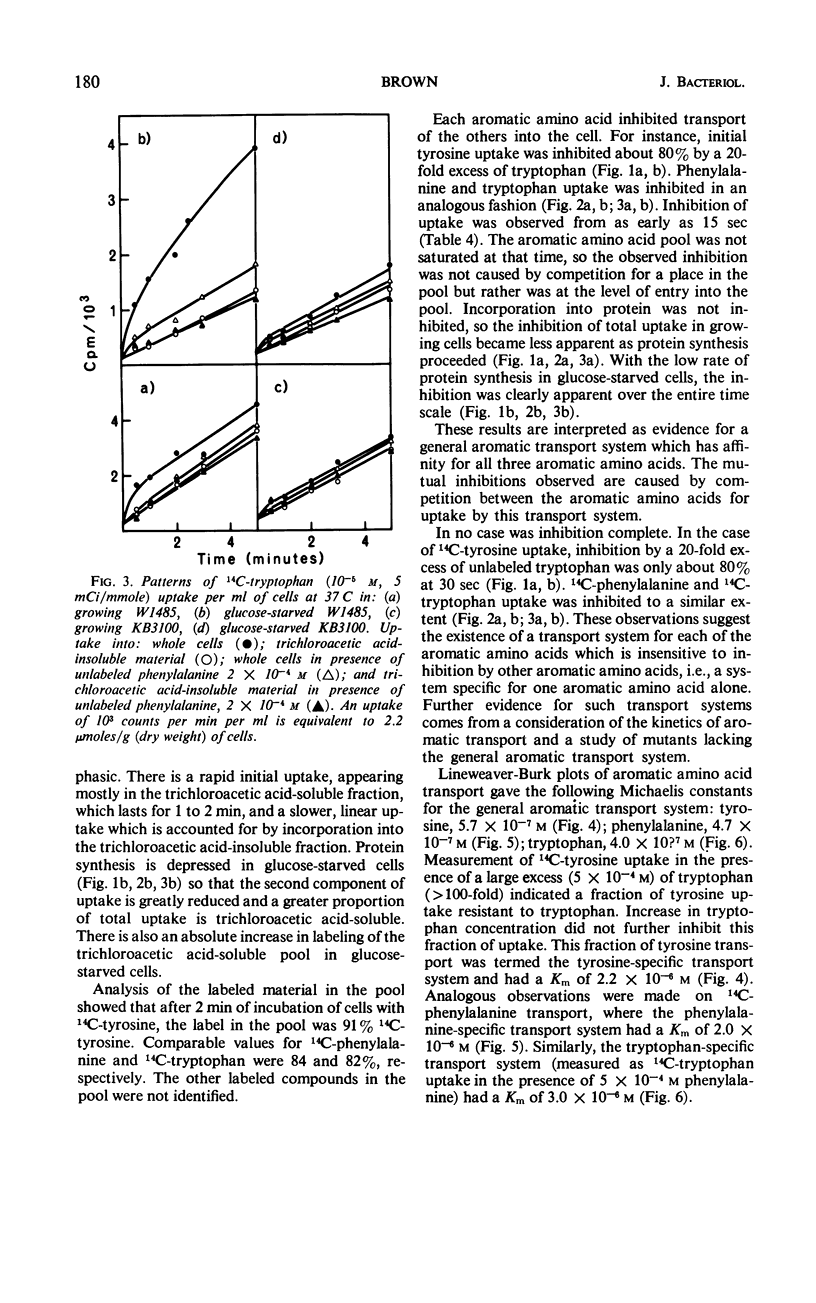
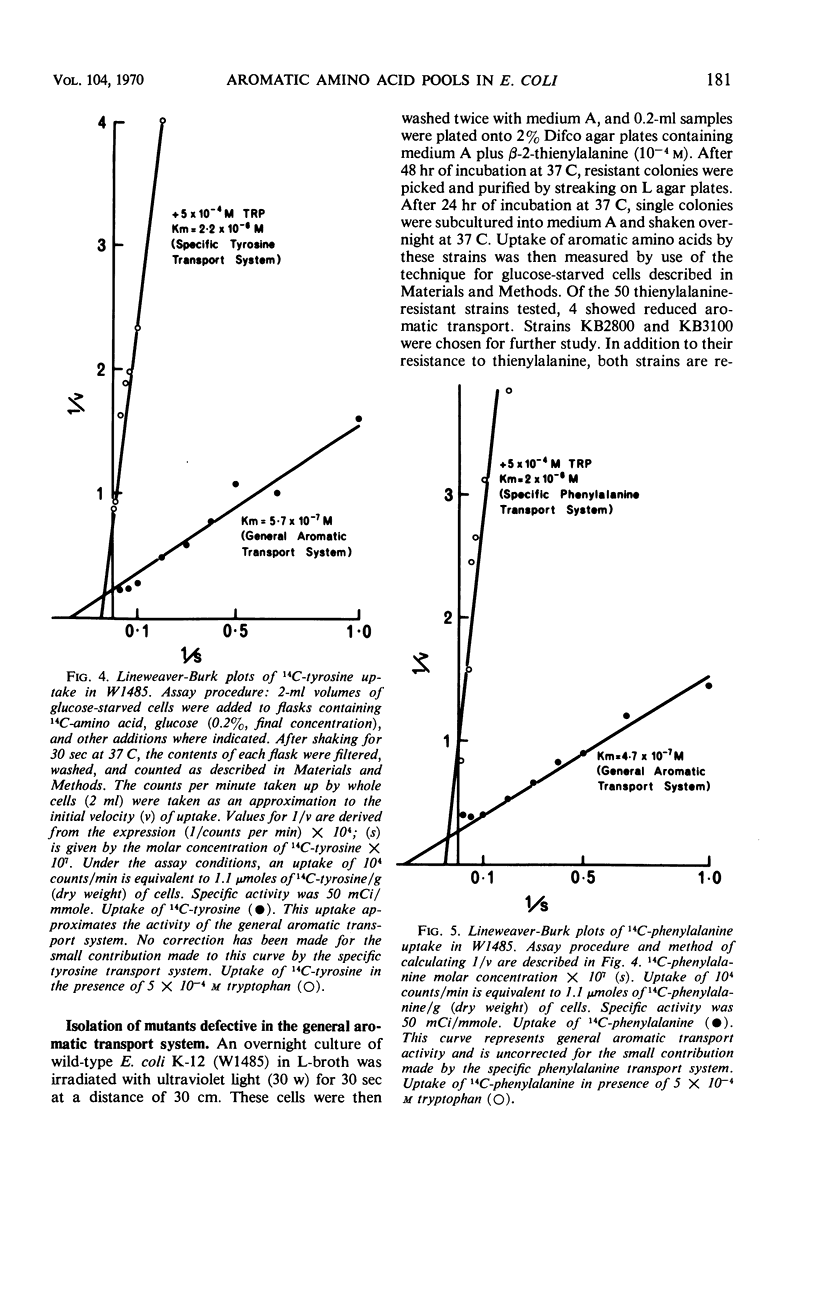
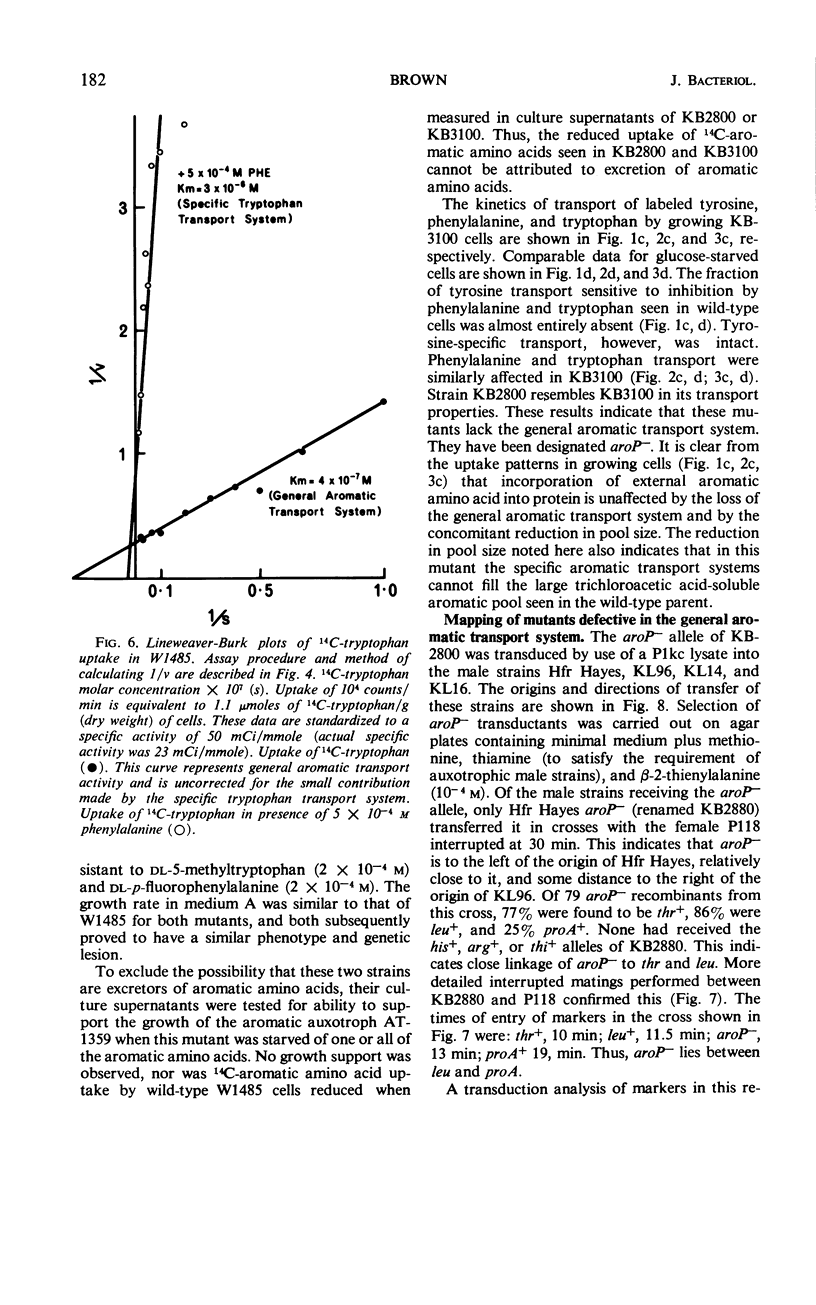
Phenylalanine, tyrosine, and tryptophan were taken up into cells of Escherichia coli K-12 by a general aromatic transport system. Apparent Michaelis constants for the three amino acids were 4.7 × 10−7, 5.7 × 10−7, and 4.0 × 10−7m, respectively. High concentrations (> 0.1 mm) of histidine, leucine, methionine, alanine, cysteine, and aspartic acid also had an affinity for this system. Mutants lacking the general aromatic transport system were resistant to p-fluorophenylalanine, β-2-thienylalanine, and 5-methyltryptophan. They mapped at a locus, aroP, between leu and pan on the chromosome, being 30% cotransducible with leu and 43% cotransducible with pan. Phenylalanine, tyrosine, and tryptophan were also transported by three specific transport systems. The apparent Michaelis constants of these systems were 2.0 × 10−6, 2.2 × 10−6, and 3.0 × 10−6m, respectively. An external energy source, such as glucose, was not required for activity of either general or specific aromatic transport systems. Azide and 2,4-dinitrophenol, however, inhibited all aromatic transport, indicating that energy production is necessary. Between 80 and 90% of the trichloroacetic acid-soluble pool formed from a particular exogenous aromatic amino acid was generated by the general aromatic transport system. This contribution was abolished when uptake was inhibited by competition by the other aromatic amino acids or by mutation in aroP. Incorporation of the former amino acid into protein was not affected by the reduction in its pool size, indicating that the general aromatic transport system is not essential for the supply of external aromatic amino acids to protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Roth J. R. Histidine and aromatic permeases of Salmonella typhimurim. J Bacteriol. 1968 Nov;96(5):1742–1749. doi: 10.1128/jb.96.5.1742-1749.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROUS S. E., DEMOSS R. D. STUDIES ON TRYPTOPHAN PERMEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 6;73:623–637. doi: 10.1016/0006-3002(63)90332-9. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk B. G., DeBusk A. G. Molecular transport in Neurospora crassa. I. Biochemical properties of a phenylalanine permease. Biochim Biophys Acta. 1965 Jun 15;104(1):139–150. doi: 10.1016/0304-4165(65)90229-1. [DOI] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- HAYES W. The mechanism of genetic recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1953;18:75–93. doi: 10.1101/sqb.1953.018.01.016. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Influence of carbon or nitrogen starvation on amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1969 Oct;100(1):276–282. doi: 10.1128/jb.100.1.276-282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Low recombination frequency for markers very near the origin in conjugation in E. coli. Genet Res. 1965 Nov;6(3):469–473. doi: 10.1017/s0016672300004341. [DOI] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic analysis of glutamate transport and glutamate decarboxylase in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1409–1415. doi: 10.1128/jb.93.4.1409-1415.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic analysis of the glutamate permease in Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1118–1128. doi: 10.1128/jb.97.3.1118-1128.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- SCHWARTZ J. H., MAAS W. K., SIMON E. J. An impaired concentrating mechanism for amino acids in mutants of Escherichia coli resistant to L-canavanine and D-serine. Biochim Biophys Acta. 1959 Apr;32:582–583. doi: 10.1016/0006-3002(59)90650-x. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Revised linkage map of Salmonella typhimurium. Bacteriol Rev. 1967 Dec;31(4):354–372. doi: 10.1128/br.31.4.354-372.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Nester E. W. Mutants of Escherichia coli with an altered tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1969 Oct;100(1):167–175. doi: 10.1128/jb.100.1.167-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



