Abstract
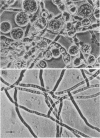
The antibiotic polyoxin D was shown to inhibit the incorporation of 14C-glucosamine into cell wall chitin in Neurospora crassa at levels which were comparable with those required for inhibition of fungal growth. At the same time, the antibiotic increased the accumulation of a nucleotide, which was identified as uridine diphosphate (UDP)-N-acetylglucosamine, indicating inhibition of chitin synthesis. Chitin synthetase (UDP-N-acetylglucosamine: chitin N-acetylglucosaminyl transferase, EC 2.4.1.16) of N. crassa was found to be strongly inhibited by polyoxin D, as determined by the transfer of 14C-N-acetylglucosamine from 14C-UDP-N-acetylglucosamine to the particulate fraction. The inhibition was competitive with respect to UDP-N-acetylglucosamine and specific for chitin synthetase. The Ki for polyoxin D in the reaction was 1.40 × 10−6m, and the Km for UDP-N-acetylglucosamine was 1.43 × 10−3m. The formation of osmotically sensitive, protoplast-like structures, when the fungus Cochliobolus miyabeanus was grown in the presence of polyoxin D, also suggested that the primary site of action of polyoxin D was in the formation of cell wall structures.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUMENTHAL H. J., ROSEMAN S. Quantitative estimation of chitin in fungi. J Bacteriol. 1957 Aug;74(2):222–224. doi: 10.1128/jb.74.2.222-224.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CABIB E., LELOIR L. F., CARDINI C. E. Uridine diphosphate acetylglucosamine. J Biol Chem. 1953 Aug;203(2):1055–1070. [PubMed] [Google Scholar]
- Camargo E. P., Dietrich C. P., Sonneborn D., Strominger J. L. Biosynthesis of chitin in spores and growing cells of Blastocladiella emersonii. J Biol Chem. 1967 Jul 10;242(13):3121–3128. [PubMed] [Google Scholar]
- Endo A., Misato T. Polyoxin D, a competitive inhibitor of UDP-N-acetylglucosamine: chitin N-acetylglucosaminyltransferase in Neurospora crassa. Biochem Biophys Res Commun. 1969 Nov 6;37(4):718–722. doi: 10.1016/0006-291x(69)90870-5. [DOI] [PubMed] [Google Scholar]
- GLASER L., BROWN D. H. The synthesis of chitin in cell-free extracts of Neurospora crassa. J Biol Chem. 1957 Oct;228(2):729–742. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- STROMINGER J. L., SMITH M. S. Uridine diphosphoacetylglucosamine pyrophosphorylase. J Biol Chem. 1959 Jul;234(7):1822–1827. [PubMed] [Google Scholar]
- SUZUKI S., ISONO K., NAGATSU J., MIZUTANI T., KAWASHIMA Y., MIZUNO T. A NEW ANTIBIOTIC, POLYOXIN A. J Antibiot (Tokyo) 1965 May;18:131–131. [PubMed] [Google Scholar]
- Scher M., Lennarz W. J., Sweeley C. C. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner W. A lipid intermediate in mannan biosynthesis in yeast. Biochem Biophys Res Commun. 1969 Apr 10;35(1):144–150. doi: 10.1016/0006-291x(69)90496-3. [DOI] [PubMed] [Google Scholar]
- Weiner I. M., Higuchi T., Rothfield L., Saltmarsh-Andrew M., Osborn M. J., Horecker B. L. Biosynthesis of bacterial lipopolysaccharide. V. Lipid-linked intermediates in the biosynthesis of the O-antigen groups of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1965 Jul;54(1):228–235. doi: 10.1073/pnas.54.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]