Abstract
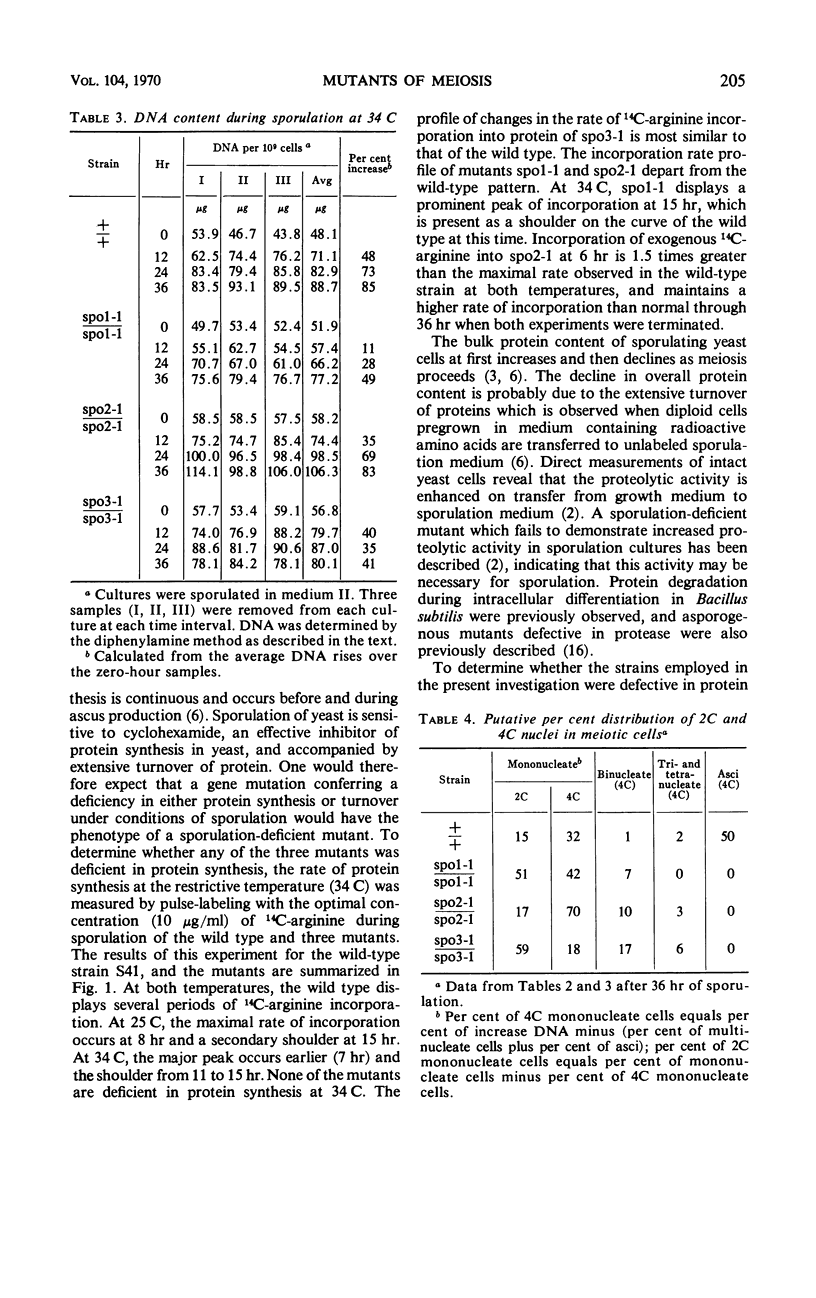
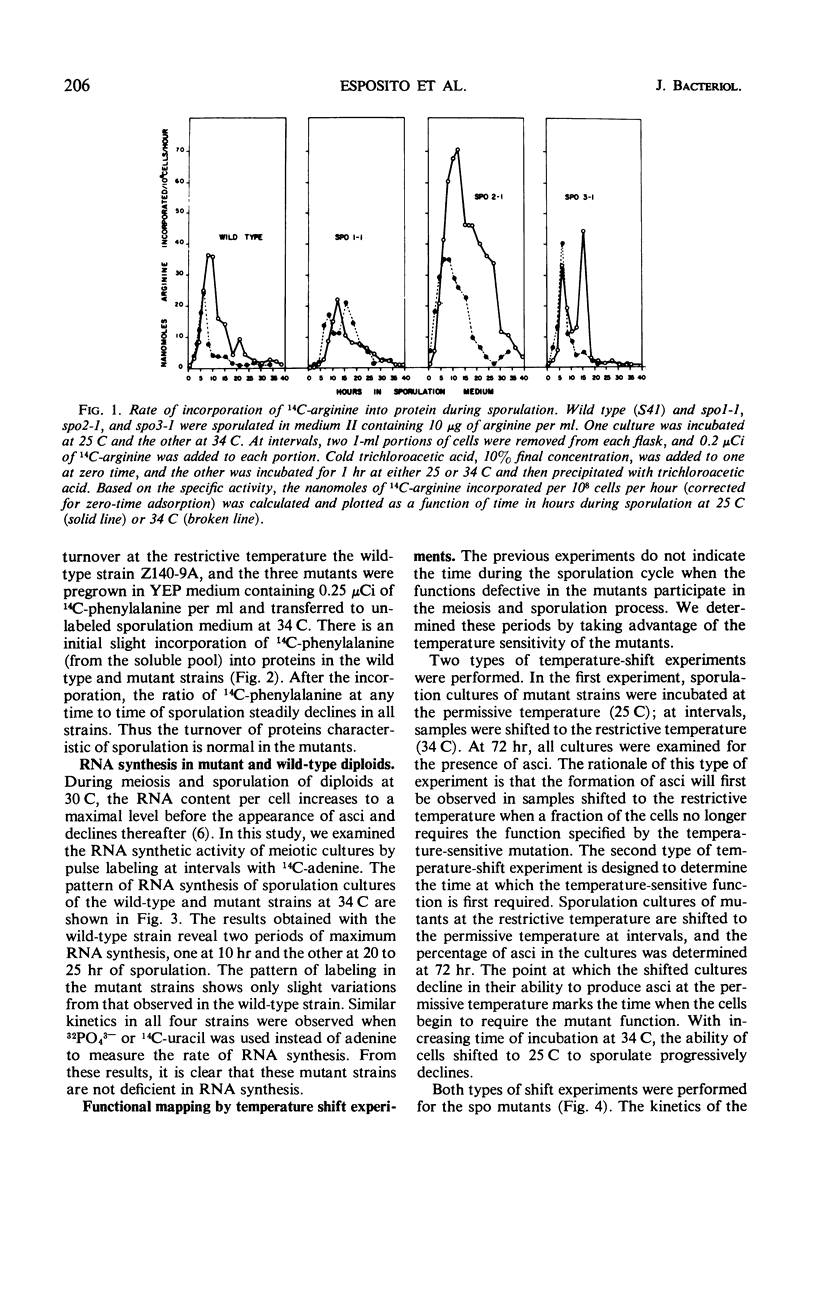
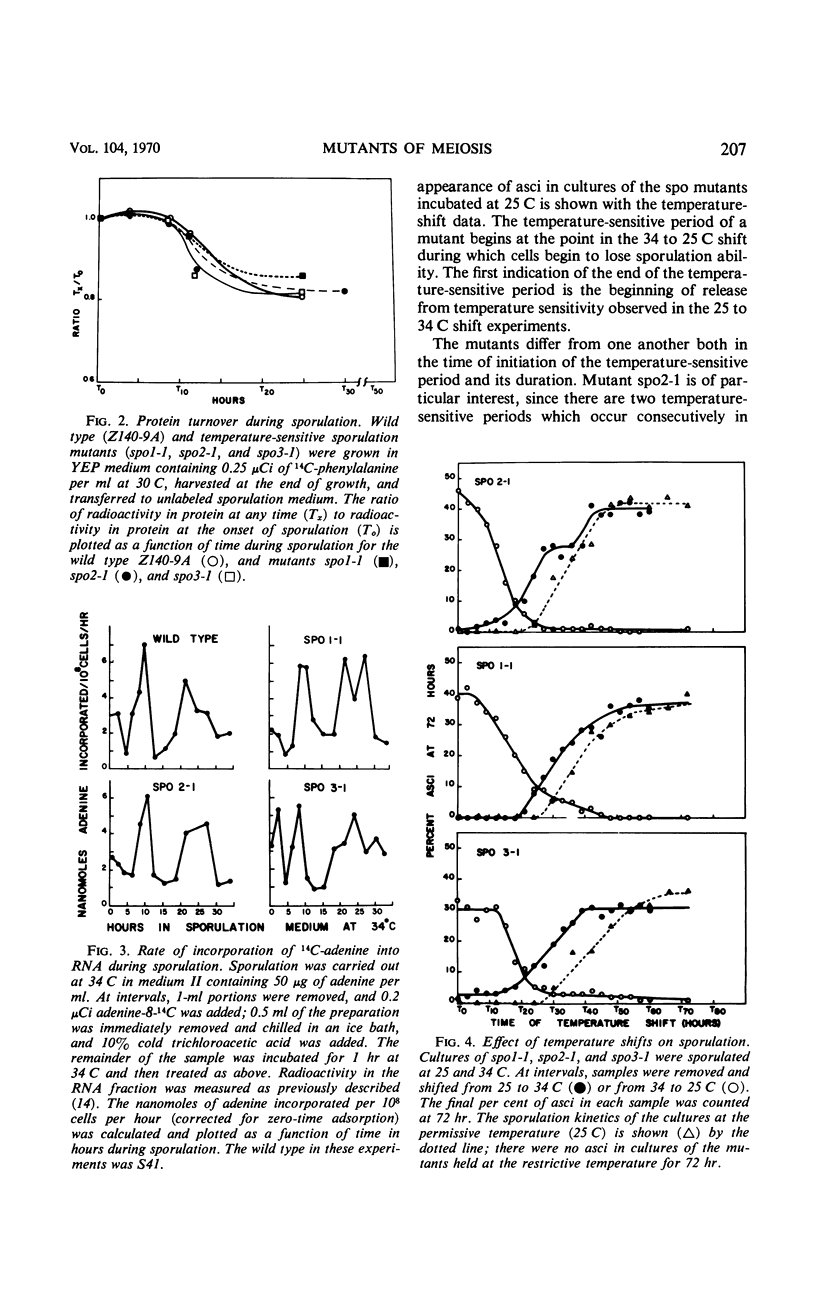
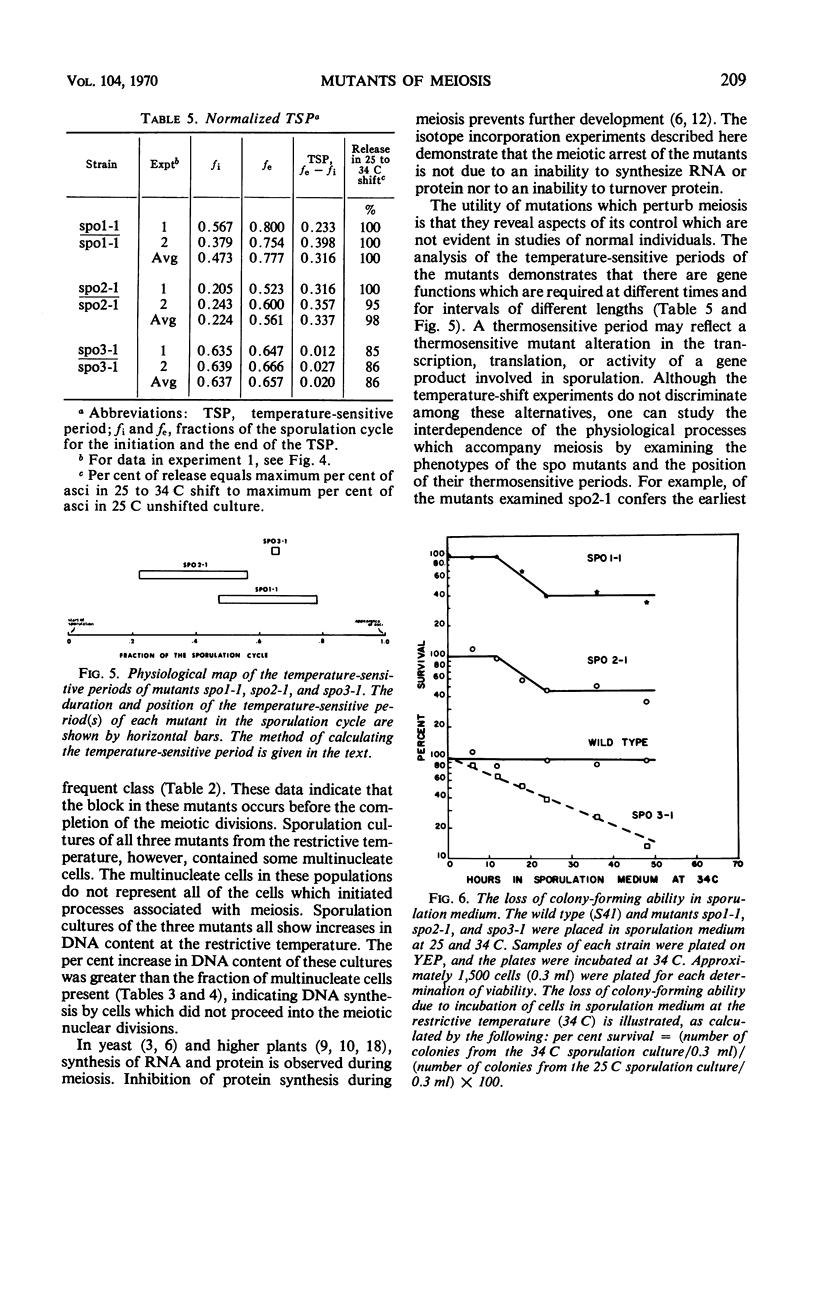
Three temperature-sensitive mutants, spo1-1, spo2-1, and spo3-1, were characterized with respect to their behavior in sporulation medium at a restrictive temperature. The time of expression of the functions defective in the mutants was determined by temperature-shift experiments during the sporulation process. In addition, each mutant was examined for the following: (i) its ability to undergo the nuclear divisions of meiosis; (ii) deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and protein synthesis; (iii) protein turnover; and (iv) colony-forming ability after exposure to sporulation medium. Mutant spo1-1 is defective in a function which confers a temperature-sensitive period which extends over 32% of the sporulation cycle. The temperature-sensitive period of mutant spo2-1 occupies 34% of the cycle, whereas the temperature-sensitive period of mutant spo3-1 extends over 2% of the sporulation cycle. Cytological evidence indicates that all three mutants initiate but do not complete the meiotic nuclear divisions. The DNA content of sporulation cultures of mutants spo1-1 and spo3-1 did not increase to the wild-type level; DNA synthesis in spo2-1 was normal. All three strains exhibit a loss of colony-forming ability during incubation in sporulation medium at the restrictive temperature. RNA and protein synthesis and protein turnover occur in the mutants.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. W., Miller J. J. Proteolytic activity of intact yeast cells during sporulation. Can J Microbiol. 1968 Sep;14(9):957–963. doi: 10.1139/m68-159. [DOI] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E. The genetic control of sporulation in Saccharomyces. I. The isolation of temperature-sensitive sporulation-deficient mutants. Genetics. 1969 Jan;61(1):79–89. doi: 10.1093/genetics/61.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANESAN A. T., HOLTER H., ROBERTS C. Some observations on sporulation in Saccharomyces. C R Trav Lab Carlsberg Chim. 1958;31(1):1–6. [PubMed] [Google Scholar]
- HOTTA Y., STERN H. SYNTHESIS OF MESSENGER-LIKE RIBONUCLEIC ACID AND PROTEIN DURING MEIOSIS IN ISOLATED CELLS OF TRILLIUM ERECTUM. J Cell Biol. 1963 Oct;19:45–58. doi: 10.1083/jcb.19.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Parchman L. G., Stern H. Protein synthesis during meiosis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):575–582. doi: 10.1073/pnas.60.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- Parchman L. G., Stern H. The inhibition of protein synthesis in meiotic cells and its effect on chromosome behavior. Chromosoma. 1969;26(3):298–311. doi: 10.1007/BF00326524. [DOI] [PubMed] [Google Scholar]
- Rodenberg S., Steinberg W., Piper J., Nickerson K., Vary J., Epstein R., Halvorson H. O. Relationship between protein and ribonucleic acid synthesis during outgrowth of spores of Bacillus cereus. J Bacteriol. 1968 Aug;96(2):492–500. doi: 10.1128/jb.96.2.492-500.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERMAN F., ROMAN H. Evidence for two types of allelic recombination in yeast. Genetics. 1963 Feb;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]



