Abstract
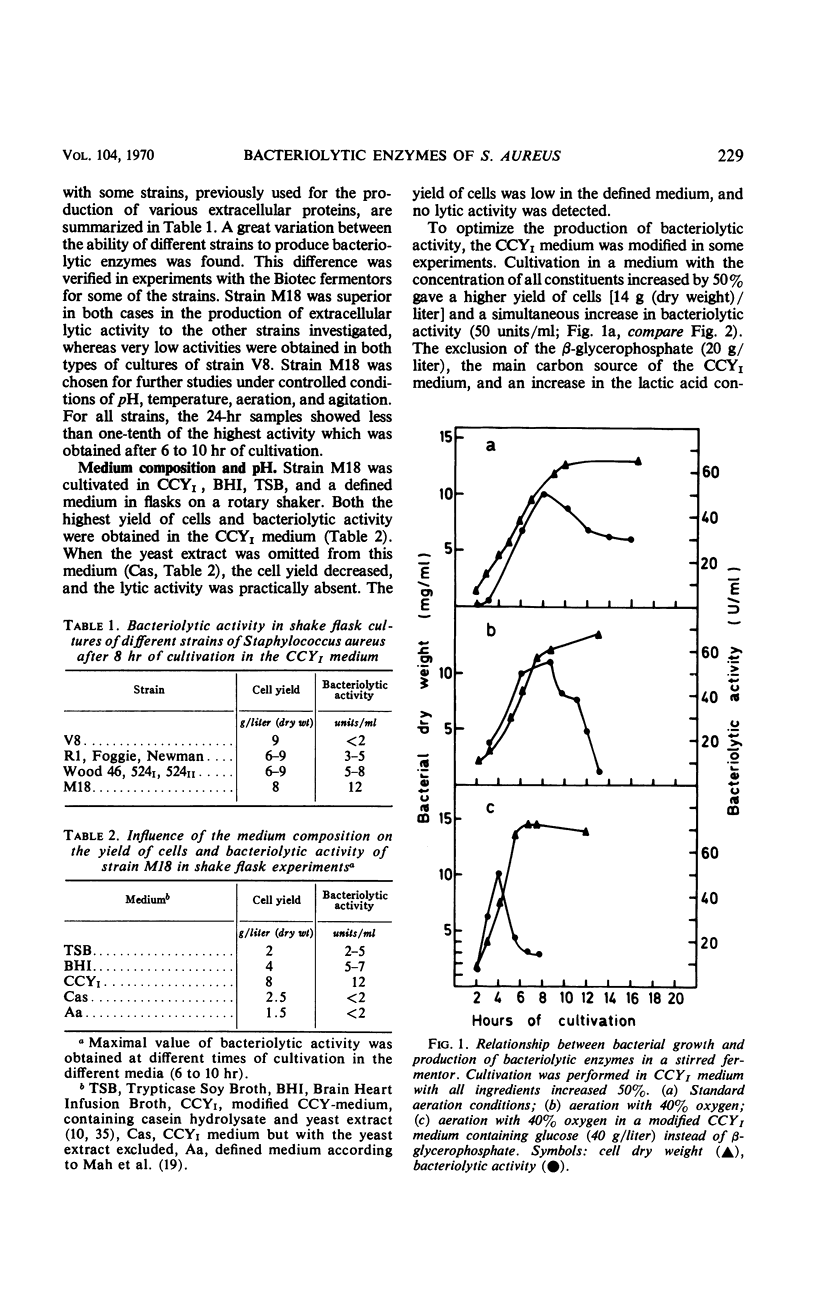
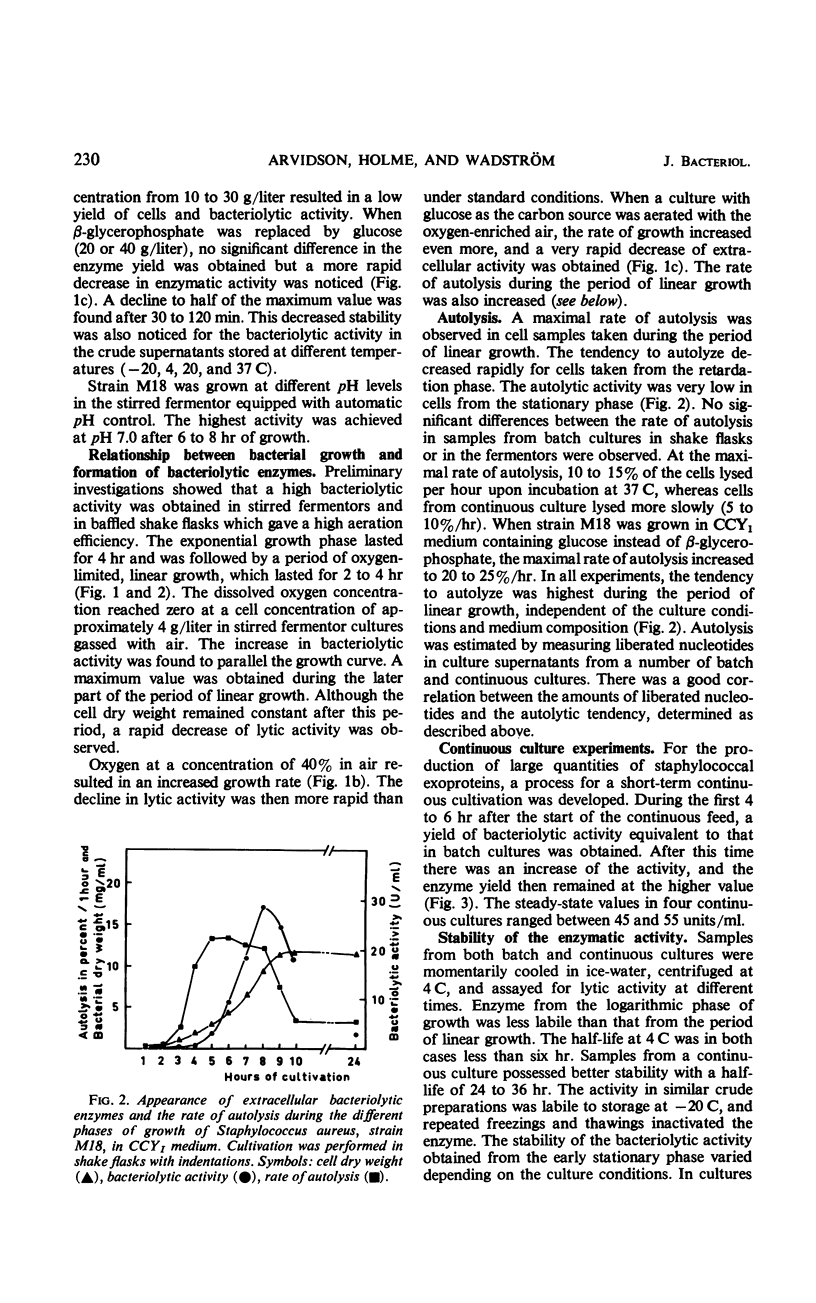
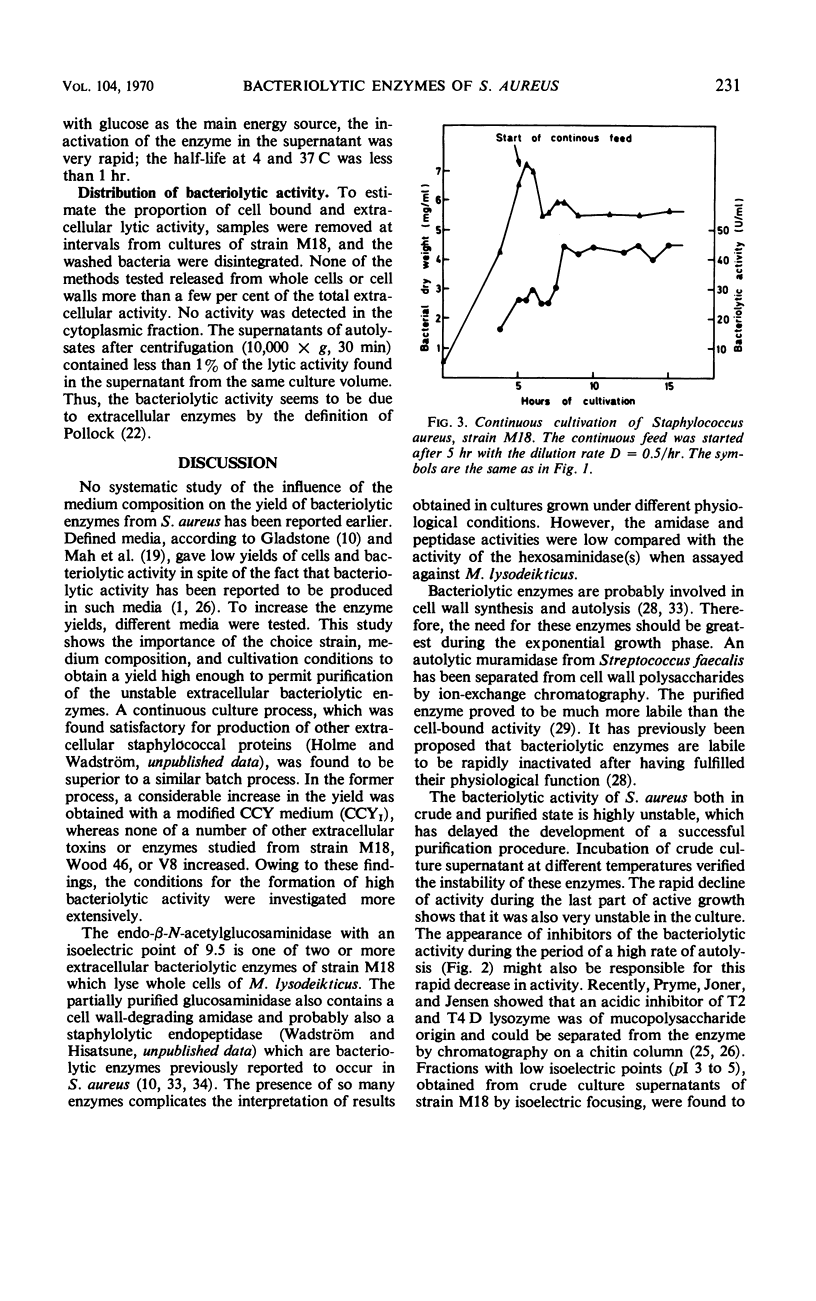
The formation of bacteriolytic enzymes of Staphylococcus aureus, with special reference to strain M18, was investigated under a variety of conditions. The bacteriolytic activity was tested by using whole cells of Micrococcus lysodeikticus as a substrate. Complex media were required for production, and a Casein Hydrolysate-Yeast Extract medium (CCYI) was superior to Brain Heart Infusion and Trypticase Soy Broth. The optimal pH level for production was 7.0. Effective oxygenation and exchange of the β-glycerophosphate of the CCYI medium for glucose increased the rates of growth and autolysis and the rate of appearance of extracellular bacteriolytic enzymes. However, the extracellular lytic activity decreased more rapidly at the end of the growth period than under the standard culture conditions. The appearance of inhibitor(s), probably derived from autolysis, might be responsible for this rapid decrease. The highest yields were obtained in a continuous process in which the activity was almost twice that of batch cultures grown under the same conditions. The bacteriolytic activity produced in continuous culture had a considerably increased stability in the purification process. The advantage of producing unstable bacterial proteins in continuous culture under controlled growth conditions is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLITTERI A., BERNARDINI A. LA PRODUZIONE DI LISOZIMA DA PARTE DELLO STAPH. AUREUS IN RAPPORTO ALLA COMPOSIZIONE DEL TERRENO DI CULTURA. Boll Soc Ital Biol Sper. 1963 Dec 31;39:1839–1843. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles N. W., Gross R. Influence of organic anions on the liberation of penicillinase from Staphylococcus aureus. Biochem J. 1967 Mar;102(3):748–752. doi: 10.1042/bj1020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles N. W., Gross R. Liberation of surface-located penicillinase from Staphylococcus aureus. Biochem J. 1967 Mar;102(3):742–747. doi: 10.1042/bj1020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover M. J., Thompson J. S., Shockman G. D. Autolytic enzyme of Streptococcus faecalis: release of soluble enzyme from cell walls. Biochem Biophys Res Commun. 1966 Jun 13;23(5):713–719. doi: 10.1016/0006-291x(66)90459-1. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., VAN HEYNINGEN W. E. Staphylococcal leucocidins. Br J Exp Pathol. 1957 Apr;38(2):123–137. [PMC free article] [PubMed] [Google Scholar]
- Grossgebauer K., Schmidt B., Langmaack H. Lysozyme production as an aid for identification of potentially pathogenic strains of staphylococci. Appl Microbiol. 1968 Nov;16(11):1745–1747. doi: 10.1128/am.16.11.1745-1747.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J. Frequency of staphylococcal lysozyme production tested by plate method. J Clin Pathol. 1968 May;21(3):390–393. doi: 10.1136/jcp.21.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J. Purification and properties of lysozyme produced by Staphylococcus aureus. J Bacteriol. 1968 Feb;95(2):376–384. doi: 10.1128/jb.95.2.376-384.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E., Silverman C. S. Lysis of Staphylococcus aureus cell walls by a soluble staphylococcal enzyme. J Bacteriol. 1968 Jan;95(1):99–106. doi: 10.1128/jb.95.1.99-106.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay J. M. Production of lysozyme by staphylococci and its correlation with three other extracellular substances. J Bacteriol. 1966 May;91(5):1804–1810. doi: 10.1128/jb.91.5.1804-1810.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWATA T., ASAKI K., TAKAGI A. Autolytic formation of spheroplasts of Bacillus megaterium after cessation of aeration. J Bacteriol. 1961 Jan;81:160–161. doi: 10.1128/jb.81.1.160-161.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- Mah R. A., Fung D. Y., Morse S. A. Nutritional requirements of Staphylococcus aureus S-6. Appl Microbiol. 1967 Jul;15(4):866–870. doi: 10.1128/am.15.4.866-870.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malveaux F. J., San Clemente C. L. Elution of loosely bound acid phosphatase from Staphylococcus aureus. Appl Microbiol. 1967 Jul;15(4):738–743. doi: 10.1128/am.15.4.738-743.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryme I. F., Joner P. E., Jensen H. B. The appearance of phage associated lysozyme in E. coli B cells immediately after infection with phage T2. Biochem Biophys Res Commun. 1969 Aug 15;36(4):676–681. doi: 10.1016/0006-291x(69)90359-3. [DOI] [PubMed] [Google Scholar]
- Pryme I. F., Joner P. E., Jensen H. B. The isolation of an inhibitor of T-even phage lysozyme from E. coli B cells. FEBS Lett. 1969 Jul;4(1):50–51. doi: 10.1016/0014-5793(69)80193-6. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. Lytic enzymes of Staphylococcus aureus 524. Biochim Biophys Acta. 1959 Feb;31(2):564–565. doi: 10.1016/0006-3002(59)90041-1. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. Properties of a lytic enzyme produced by a strain of Bacillus subtilis. Biochim Biophys Acta. 1959 May;33(1):92–101. doi: 10.1016/0006-3002(59)90501-3. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeaton J. R., Elliott W. H. Isolation and properties of a specific bacterial ribonuclease inhibitor. Biochim Biophys Acta. 1967;145(3):547–560. doi: 10.1016/0005-2787(67)90115-3. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., LEUTGEB W. Autolytic enzymes as a source of error in the preparation and study of gram-negative cell walls. J Gen Microbiol. 1963 Jan;30:127–130. doi: 10.1099/00221287-30-1-127. [DOI] [PubMed] [Google Scholar]
- WELSCH M., SALMON J. Quelques aspects de la staphylolyse. Ann Inst Pasteur (Paris) 1950 Nov;79(5):802–813. [PubMed] [Google Scholar]
- WOODIN A. M. Fractionation of a leucocidin from Staphylococcus aureus. Biochem J. 1959 Oct;73:225–237. doi: 10.1042/bj0730225. [DOI] [PMC free article] [PubMed] [Google Scholar]



