Abstract
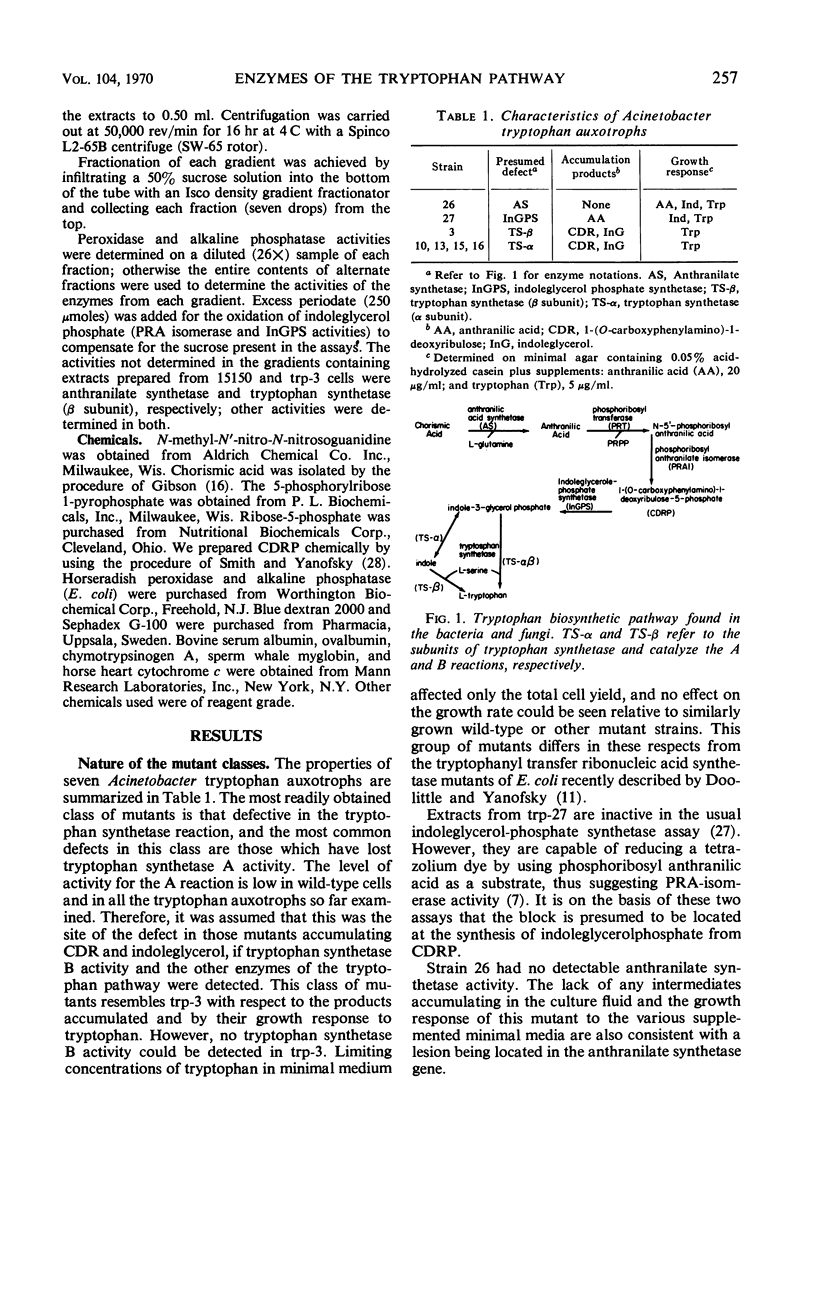
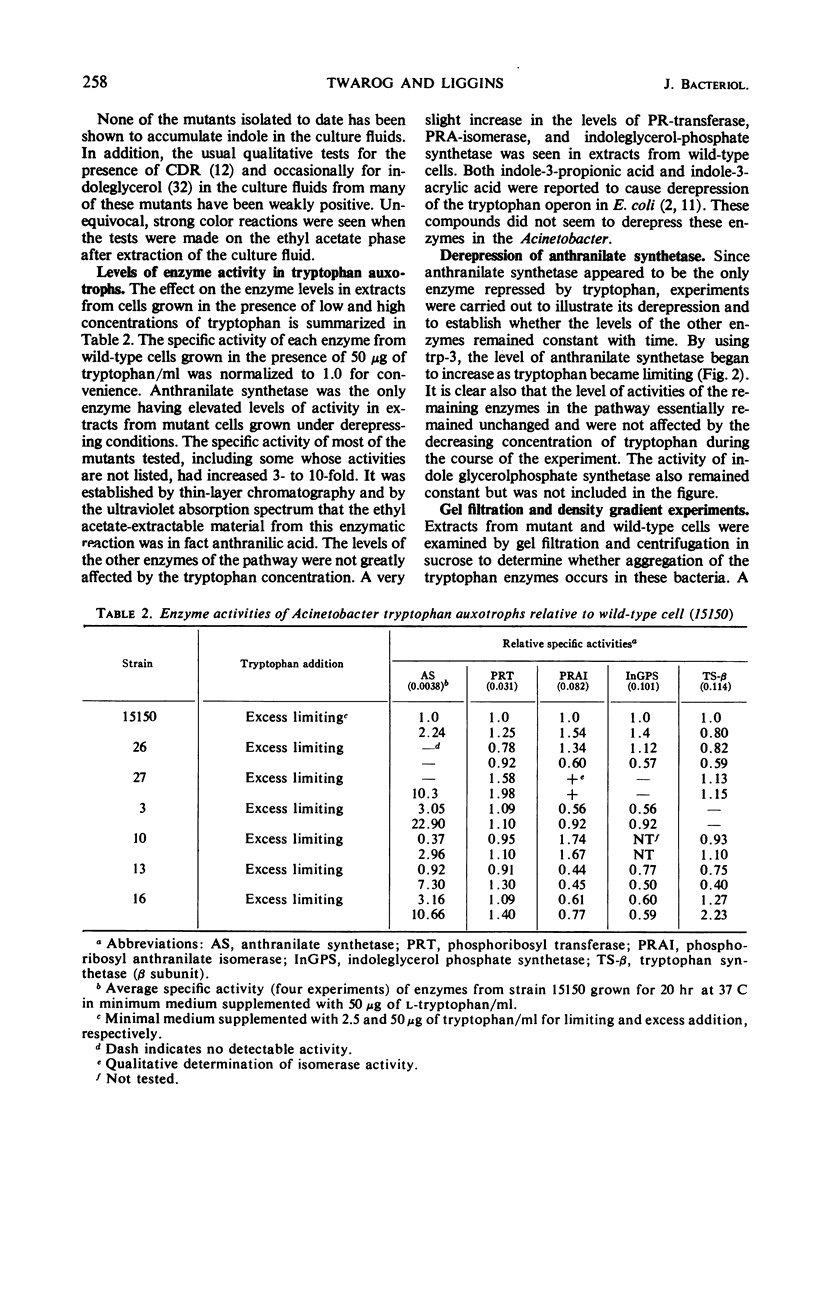
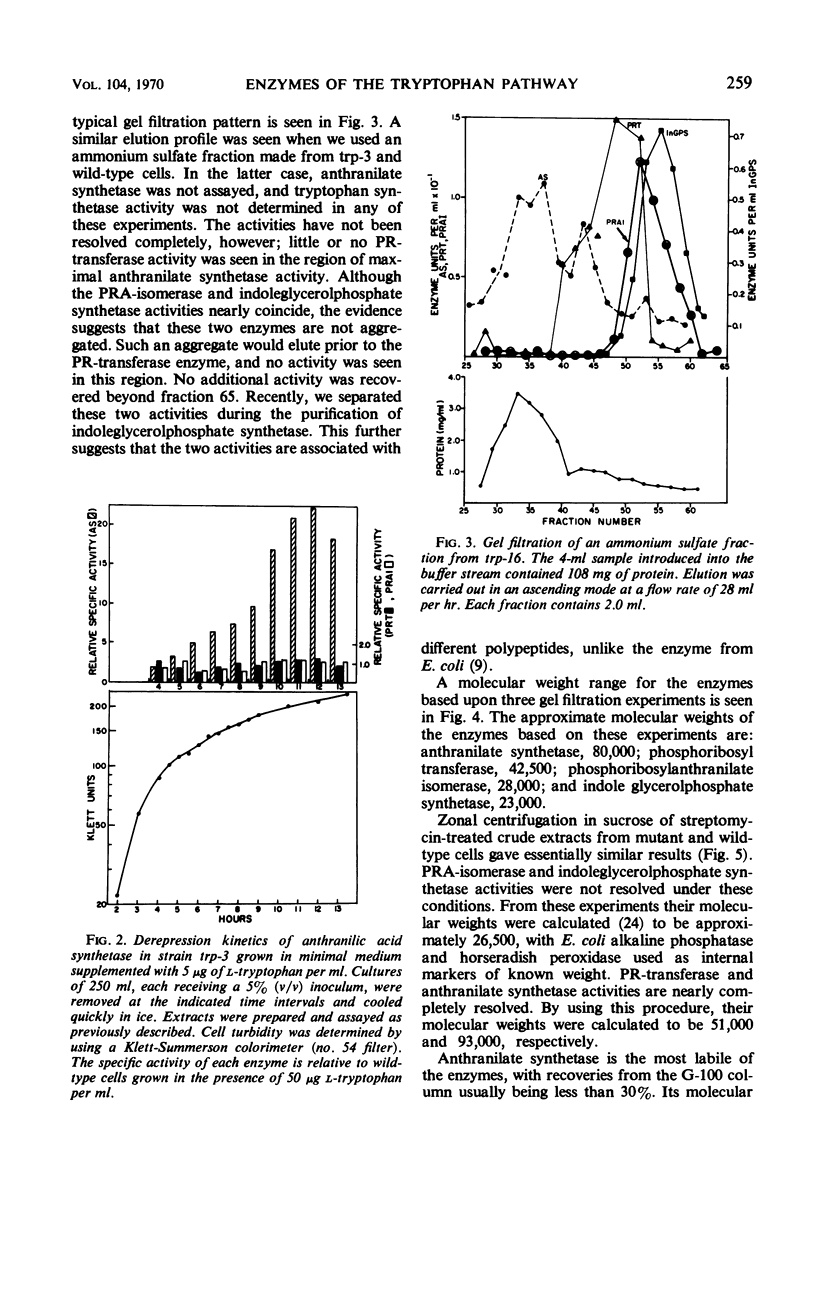
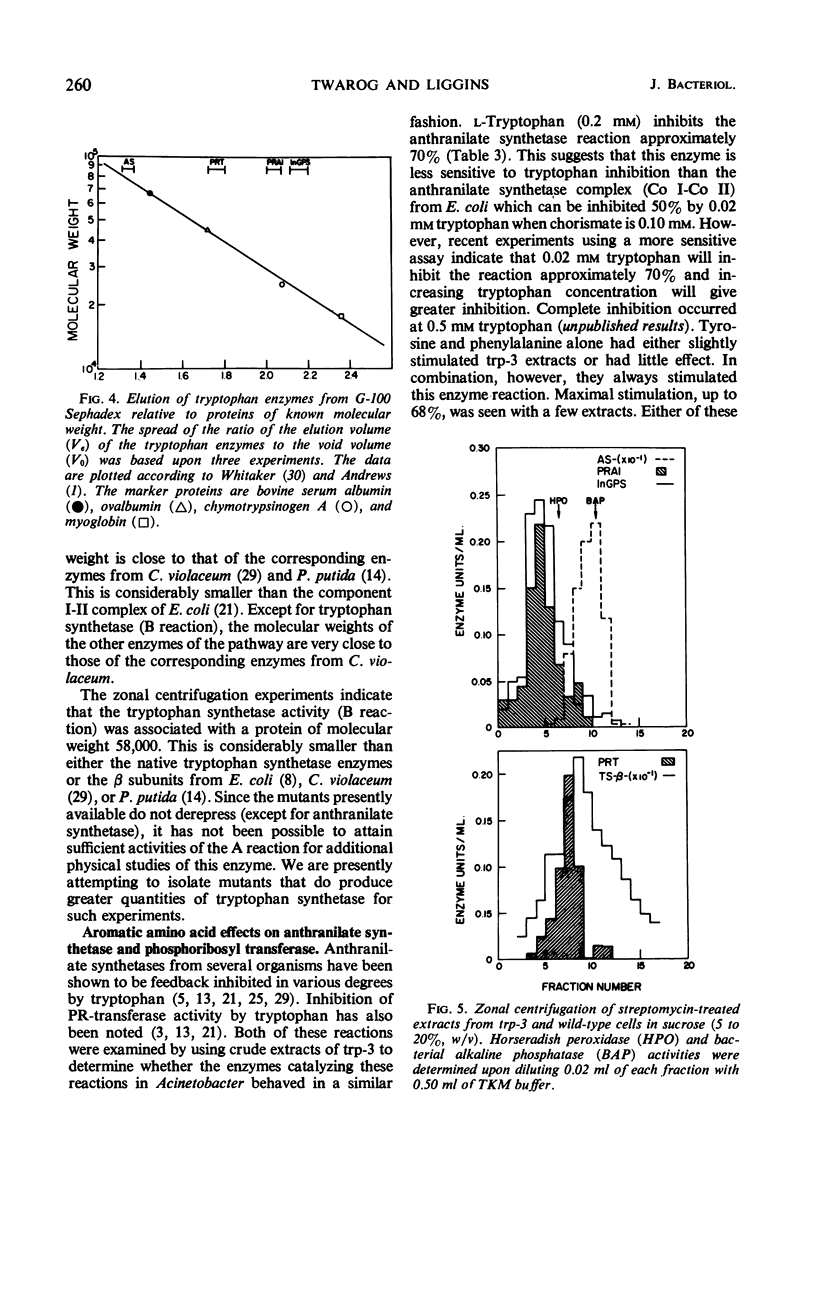
All enzymes of the tryptophan synthetic pathway were detectable in extracts from wild-type Acinetobacter calco-aceticus. The levels of these enzymes were determined in extracts from a number of auxotrophs grown under limiting tryptophan. In each case only anthranilate synthetase was found to be present in increased amounts, whereas the specific activities of the remaining enzymes remained unchanged and unaffected by the tryptophan concentration. Derepression of anthranilate synthetase was found to occur as the concentration of tryptophan became limiting. Anthranilate synthetase and phosphoribosyl transferase activities are both feedback-inhibited by tryptophan. Molecular weight determination carried out by gel filtration and zonal centrifugation in sucrose revealed that all the enzymes are less than 100,000, and no molecular aggregates of these enzymes were detected. The data indicate that tryptophan synthesis in Acinetobacter is regulated both by feedback inhibition of the first two enzymes of the pathway and by repression control of anthranilate synthetase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. F., Yanofsky C. Direction of in vivo degradation of a messenger RNA. Nature. 1968 Jul 6;219(5149):26–29. doi: 10.1038/219026a0. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. The nonenzymatic preparation in solution of N-(5'-phosphoribosyl) anthranilic acid, an intermediate in tryptophan biosynthesis. J Biol Chem. 1968 Nov 10;243(21):5605–5609. [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Association of the alpha and beta-2 subunits of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):980–990. [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616–4624. [PubMed] [Google Scholar]
- DATTA P., GEST H. ALTERNATIVE PATTERNS OF END-PRODUCT CONTROL IN BIOSYNTHESIS OF AMINO-ACIDS OF THE ASPARTIC FAMILY. Nature. 1964 Sep 19;203:1259–1261. doi: 10.1038/2031259a0. [DOI] [PubMed] [Google Scholar]
- DOY C. H., GIBSON F. 1-(o-Carboxyphenylamino)-1-deoxyribulose. A compound formed by mutant strains of Aerobacter aerogenes and Escherichia coli blocked in the biosynthesis of tryptophan. Biochem J. 1959 Aug;72:586–597. doi: 10.1042/bj0720586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatsu T., Crawford I. P. Enzymes of the tryptophan synthetic pathway in Pseudomonas putida. J Bacteriol. 1968 Jan;95(1):107–112. doi: 10.1128/jb.95.1.107-112.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Hutchinson M. A., Belser W. L. Enzymes of tryptophan biosynthesis in Serratia marcescens. J Bacteriol. 1969 Apr;98(1):109–115. doi: 10.1128/jb.98.1.109-115.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Organization of the tryptophan pathway: a phylogenetic study of the fungi. J Bacteriol. 1967 Dec;94(6):1896–1907. doi: 10.1128/jb.94.6.1896-1907.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: Comparative studies on the complex and the subunits. J Bacteriol. 1969 Feb;97(2):734–742. doi: 10.1128/jb.97.2.734-742.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. The nature of the anthranilic acid synthetase complex of Escherichia coli. J Biol Chem. 1966 Sep 10;241(17):4112–4114. [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A., Nasser D. S. Regulation of enzyme synthesis in the aromatic amino acid pathway of Bacillus subtilus. J Bacteriol. 1969 Jan;97(1):83–90. doi: 10.1128/jb.97.1.83-90.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman J., Crawford I. P. Tryptophan synthetic pathway and its regulation in Chromobacterium violaceum. J Bacteriol. 1968 Jun;95(6):2325–2335. doi: 10.1128/jb.95.6.2325-2335.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C. The enzymatic conversion of anthranilic acid to indole. J Biol Chem. 1956 Nov;223(1):171–184. [PubMed] [Google Scholar]



