Abstract
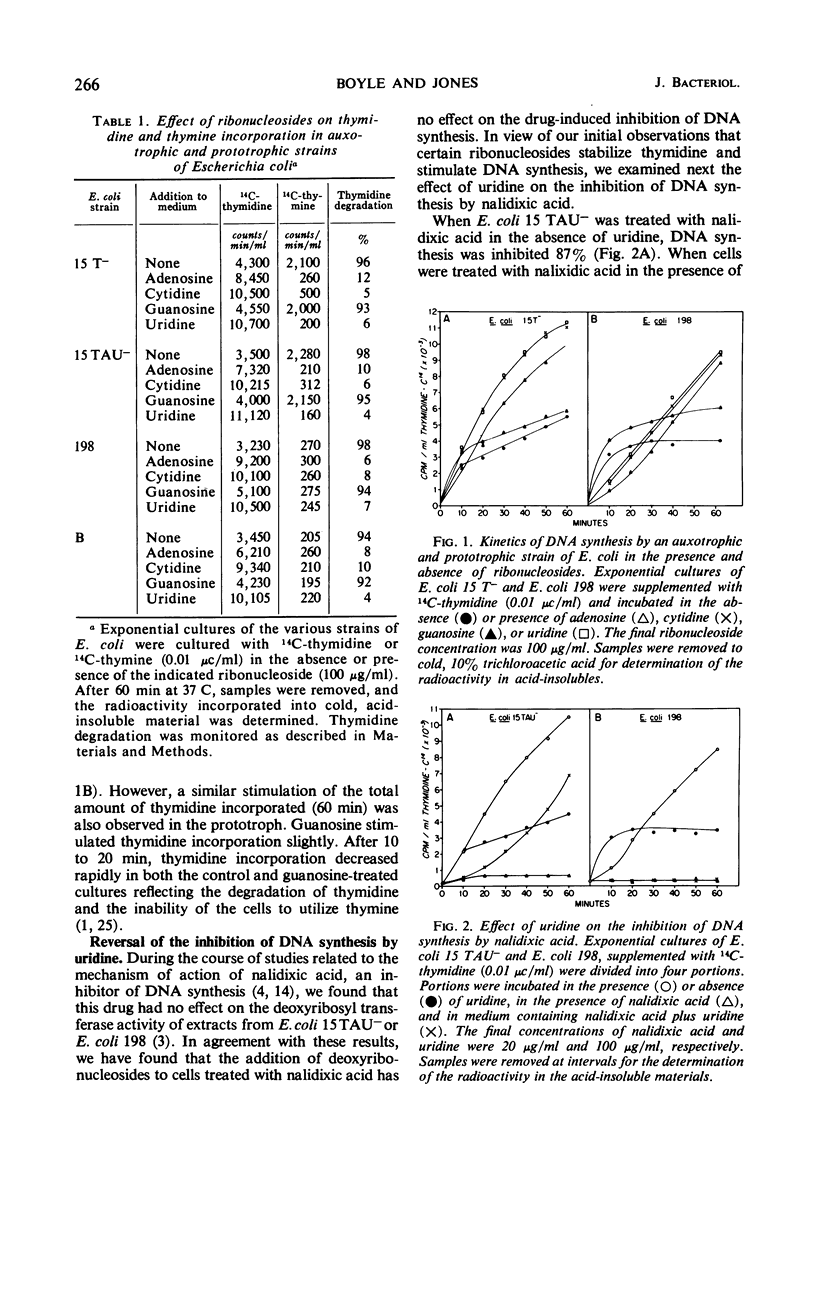
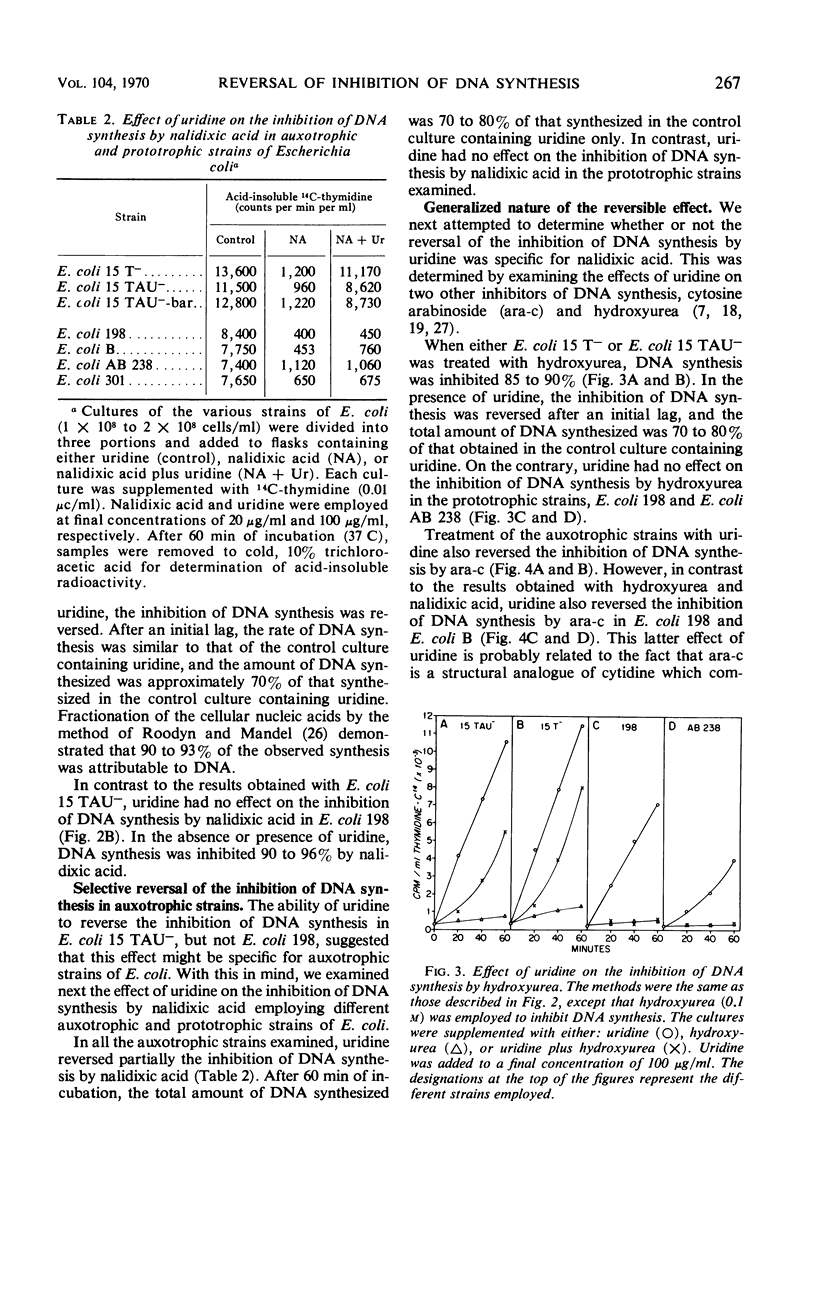
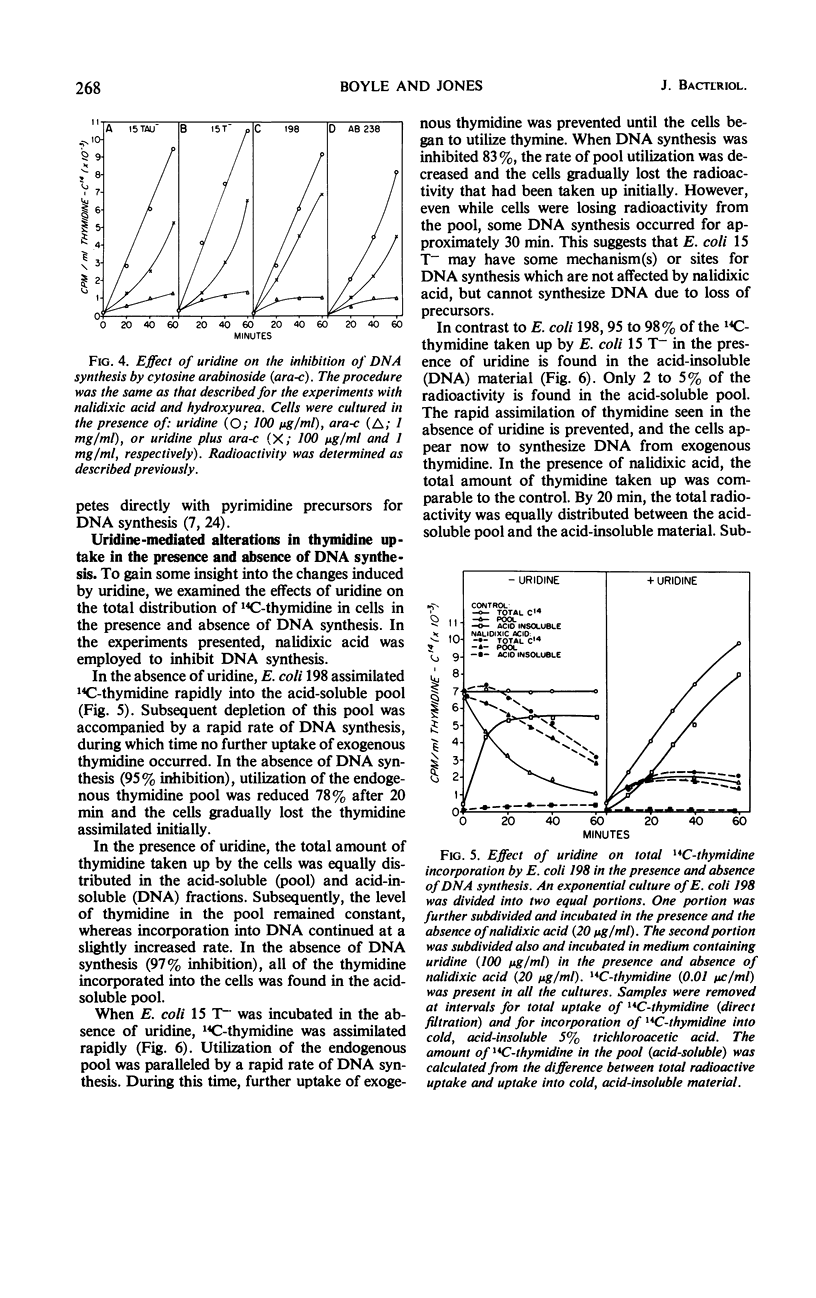
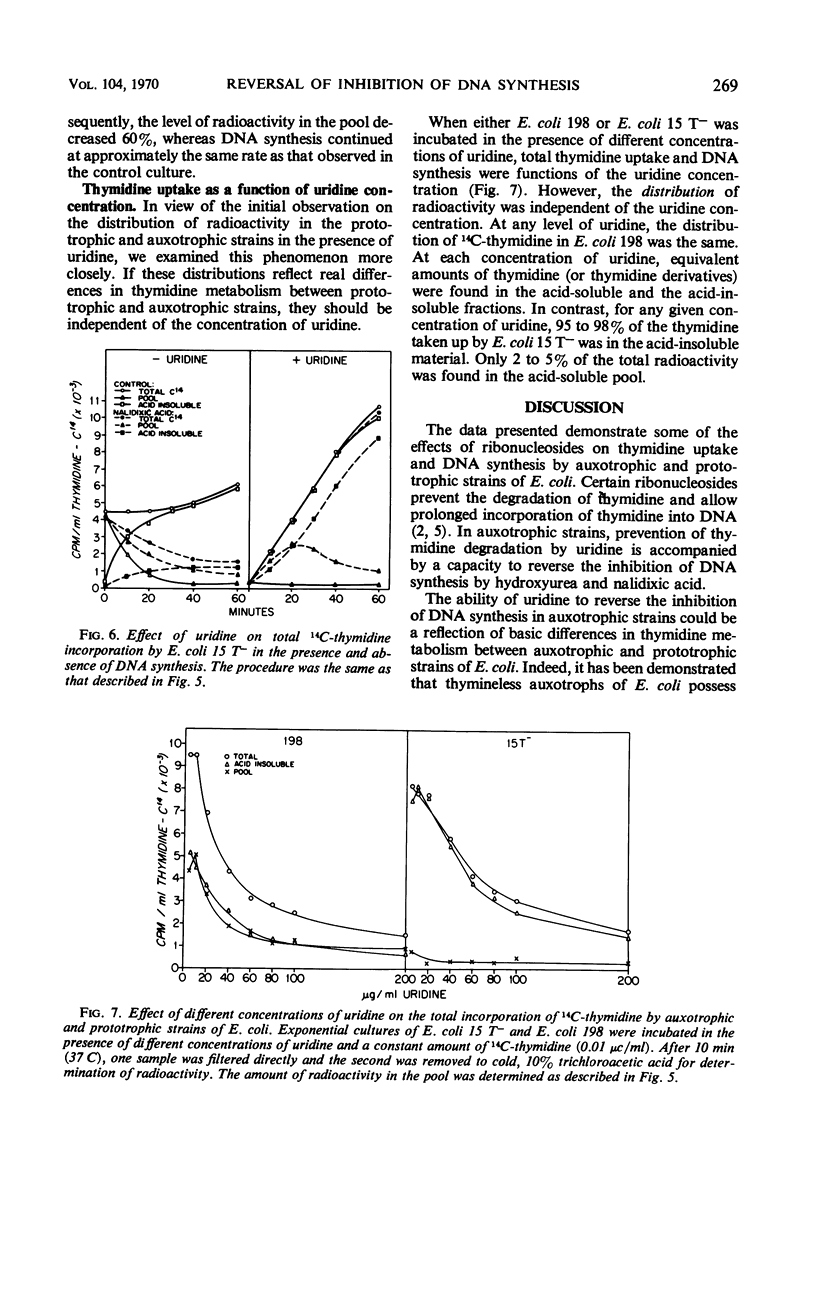
Addition of certain ribonucleosides to exponentially growing cultures of Escherichia coli increased the extent of thymidine incorporation. The prolonged uptake of thymidine was correlative with the ability of these ribonucleosides to prevent the degradation of thymidine. In addition to protecting thymidine, uridine reversed partially (70 to 80%) the inhibition of deoxyribonucleic acid (DNA) synthesis in thymineless auxotrophs by cytosine arabinoside, hydroxyurea, and nalidixic acid. This reversal was selective for auxotrophic strains since no reversal of inhibition by uridine was observed in any of the prototrophic strains examined. In the presence of uridine, the rapid assimilation of thymidine by prototrophic and auxotrophic strains was prevented and the rate of DNA synthesis became a function of the available exogenous thymidine. Under these conditions, prototrophic strains accumulated equivalent amounts of thymidine into the acid-soluble (pool) and acid-insoluble (DNA) cell fractions. In contrast, 95 to 98% of the thymidine taken up by auxotrophs was found in the acid-insoluble (DNA) cell fraction. The results suggest that different mechanisms for DNA synthesis exist in auxotrophs and prototrophs. Based on these observed differences, some possible mechanisms for the selective reversal of the inhibition of DNA synthesis in auxotrophs are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODMER W. F., GRETHER S. UPTAKE AND INCORPORATION OF THYMINE, THYMIDINE, URACIL, URIDINE, AND 5-FLUOROURACIL INTO THE NUCLEIC ACIDS OF BACILLUS SUBTILIS. J Bacteriol. 1965 Apr;89:1011–1014. doi: 10.1128/jb.89.4.1011-1014.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. V., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. Vi. Cell-free studies. J Bacteriol. 1969 Jan;97(1):230–236. doi: 10.1128/jb.97.1.230-236.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. V., Goss W. A., Cook T. M. Induction of excessive deoxyribonucleic acid synthesis in Escherichia coli by nalidixic acid. J Bacteriol. 1967 Nov;94(5):1664–1671. doi: 10.1128/jb.94.5.1664-1671.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D. R., Pardee A. B. Thymidine and thymine incorporation into deoxyribonucleic acid: inhibition and repression by uridine of thymidine phosphorylase of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1546–1550. doi: 10.1128/jb.94.5.1546-1550.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU M. Y., FISCHER G. A. A proposed mechanism of action of 1-beta-D-arabinofuranosyl-cytosine as an inhibitor of the growth of leukemic cells. Biochem Pharmacol. 1962 Jun;11:423–430. doi: 10.1016/0006-2952(62)90225-3. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V. Thymine metabolism in strains of Escherichia coli. Biochim Biophys Acta. 1958 Nov;30(2):428–429. doi: 10.1016/0006-3002(58)90071-4. [DOI] [PubMed] [Google Scholar]
- Cannon W. D., Jr, Breitman T. R. Control of deoxynucleotide biosynthesis in Escherichia coli. I. Decrease of pyrimidine deoxynucleotide biosynthesis in vivo in the presence of deoxythymidylate. Biochemistry. 1967 Mar;6(3):810–816. doi: 10.1021/bi00855a022. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskocil J., Paces V. The effect of nucleosides on the phosphorolysis of thymidine by normal and phage-infected cells of E. coli. Biochem Biophys Res Commun. 1968 Jan 25;30(2):153–158. doi: 10.1016/0006-291x(68)90463-4. [DOI] [PubMed] [Google Scholar]
- FINK K., CLINE R. E., HENDERSON R. B., FINK R. M. Metabolism of thymine (methyl-C14 or -2-C14) by rat liver in vitro. J Biol Chem. 1956 Jul;221(1):425–433. [PubMed] [Google Scholar]
- Fangman W. L. Specificity and efficiency of thymidine incorporation in Escherichia coli lacking thymidine phosphorylase. J Bacteriol. 1969 Sep;99(3):681–687. doi: 10.1128/jb.99.3.681-687.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Technique for starvation of Escherichia coli of thymine. J Bacteriol. 1965 Oct;90(4):1153–1154. doi: 10.1128/jb.90.4.1153-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammen H. O., Strand M. Thymine metabolism in Escherichia coli. II. Altered uptake of thymine after bacteriophage infection. J Biol Chem. 1967 Apr 25;242(8):1854–1863. [PubMed] [Google Scholar]
- Karlström O., Larsson A. Significance of ribonucleotide reduction in the biosynthesis of deoxyribonucleotides in Escherichia coli. Eur J Biochem. 1967 Dec;3(2):164–170. doi: 10.1111/j.1432-1033.1967.tb19512.x. [DOI] [PubMed] [Google Scholar]
- Krakoff I. H., Brown N. C., Reichard P. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res. 1968 Aug;28(8):1559–1565. [PubMed] [Google Scholar]
- LARK C., LARK K. G. EVIDENCE FOR TWO DISTINCT ASPECTS OF THE MECHANISM REGULATING CHROMOSOME REPLICATION IN ESCHERICHIA COLI. J Mol Biol. 1964 Oct;10:120–136. doi: 10.1016/s0022-2836(64)80032-2. [DOI] [PubMed] [Google Scholar]
- Lomax M. S., Greenberg G. R. Characteristics of the deo operon: role in thymine utilization and sensitivity to deoxyribonucleosides. J Bacteriol. 1968 Aug;96(2):501–514. doi: 10.1128/jb.96.2.501-514.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A. On the catabolism of deoxyribonucleosides in cells and cell extracts of Escherichia coli. Eur J Biochem. 1968 Nov;6(3):432–442. doi: 10.1111/j.1432-1033.1968.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen A. Thymineless mutants of Escherichia coli with deficiencies in deoxyribomutase and deoxyriboaldolase. Biochim Biophys Acta. 1968 Jun 18;161(1):279–282. doi: 10.1016/0005-2787(68)90325-0. [DOI] [PubMed] [Google Scholar]
- Neuhard J. Pyrimidine nucleotide metabolism and pathways of thymidine triphosphate biosynthesis in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1519–1527. doi: 10.1128/jb.96.5.1519-1527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I., COHEN S. S. Metabolism of pyrimidine arabinonucleosides and cyclonucleosides in Escherichia coli. J Biol Chem. 1960 Aug;235:2387–2392. [PubMed] [Google Scholar]
- RACHMELER M., GERHART J., ROSNER J. Limited thymidine uptake in Escherichia coli due to an inducible thymidine phosphorylase. Biochim Biophys Acta. 1961 Apr 29;49:222–225. doi: 10.1016/0006-3002(61)90888-5. [DOI] [PubMed] [Google Scholar]
- ROODYN D. B., MANDEL H. G. A simple membrane fractionation method for determining the distribution of radioactivity in chemical fractions of Bacillus cereus. Biochim Biophys Acta. 1960 Jun 17;41:80–88. doi: 10.1016/0006-3002(60)90371-1. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., Garro A. J., Levy J. A., Carr H. S. Studies with hydroxyurea. I. The reversible inhibition of bacterial DNA synthesis and the effect of hydroxyurea on the bactericidal action of streptomycin. Biochim Biophys Acta. 1966 Mar 21;114(3):501–515. [PubMed] [Google Scholar]
- Turner M. K., Abrams R., Lieberman I. Meso-alpha, beta-diphenylsuccinate and hydroxyurea as inhibitors of deoxycytidylate synthesis in extracts of Ehrlich ascites and L cells. J Biol Chem. 1966 Dec 25;241(24):5777–5780. [PubMed] [Google Scholar]



