Abstract
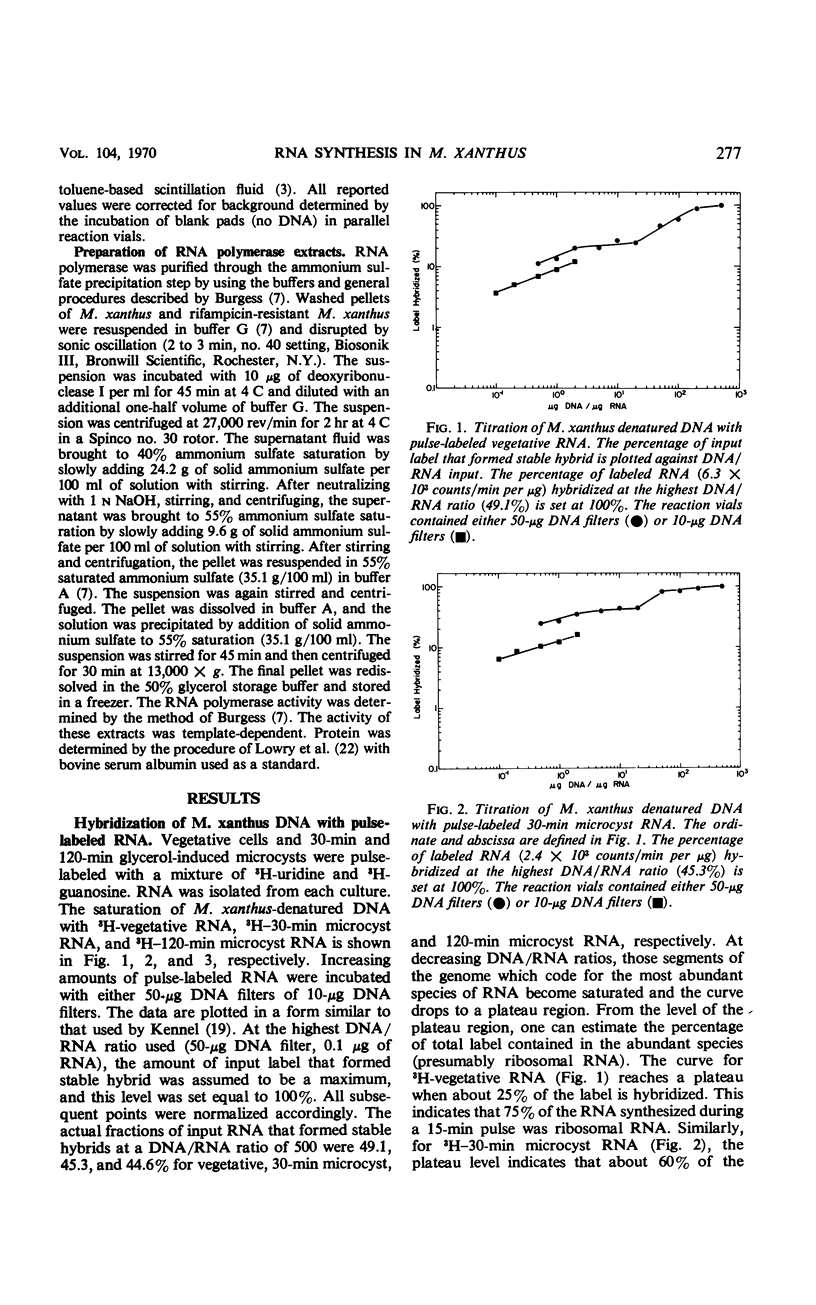
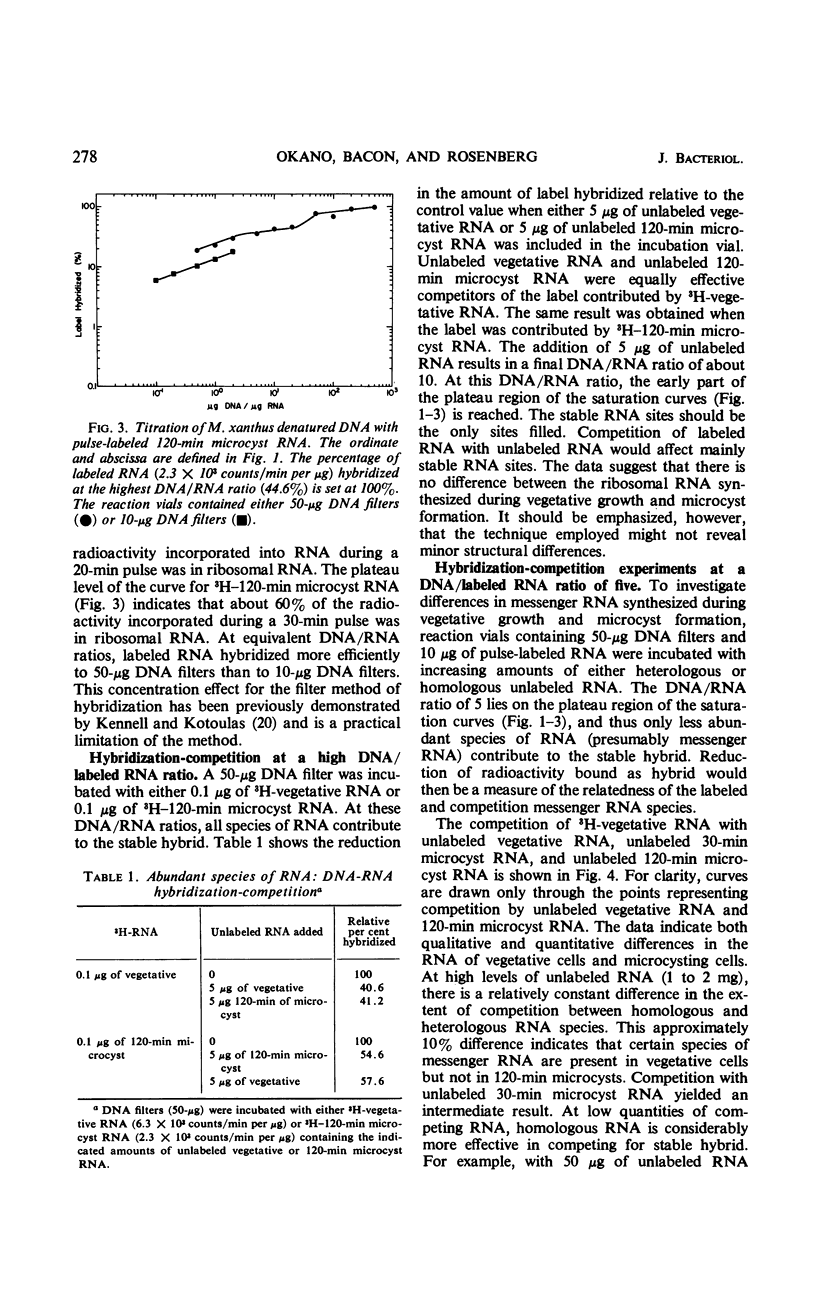
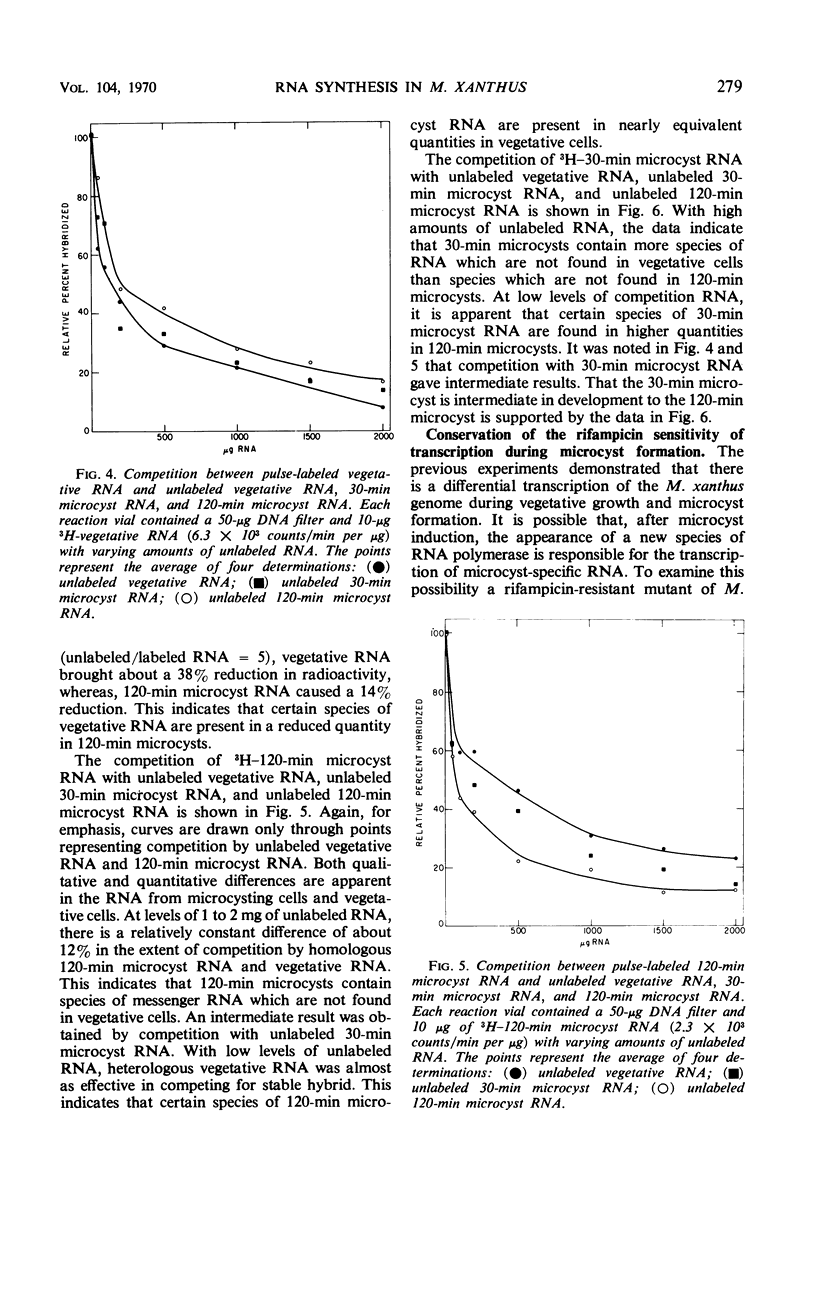
The technique of deoxyribonucleic acid-ribonucleic acid (RNA) hybridization was used to compare the RNA synthesized during vegetative growth and microcyst formation in Myxococcus xanthus. All classes of RNA, including ribosomal RNA, were synthesized during microcyst formation. The results indicate that the ribosomal RNA synthesized during microcyst formation was indistinguishable from that made during vegetative growth. Hybridization competition experiments demonstrated that certain messenger RNA species are synthesized only during vegetative growth, whereas others are synthesized only during microcyst formation. The synthesis of a new species of RNA polymerase does not appear to be responsible for differential transcription during morphogenesis in M. xanthus since the rifampicin sensitivity of transcription was conserved during microcyst formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON A. I. CHARACTERIZATION OF MESSENGER RNA IN SPORULATING BACILLUS CEREUS. J Mol Biol. 1965 Mar;11:576–588. doi: 10.1016/s0022-2836(65)80012-2. [DOI] [PubMed] [Google Scholar]
- Bacon K., Eiserling F. A. A unique structure in microcysts of Myxococcus xanthus. J Ultrastruct Res. 1967 Dec;21(5):378–382. doi: 10.1016/s0022-5320(67)80147-3. [DOI] [PubMed] [Google Scholar]
- Bacon K., Rosenberg E. Ribonucleic acid synthesis during morphogenesis in Myxococcus xanthus. J Bacteriol. 1967 Dec;94(6):1883–1889. doi: 10.1128/jb.94.6.1883-1889.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautz E. K., Bautz F. A., Dunn J. J. E. coli sigma factor: a positive control element in phage T4 development. Nature. 1969 Sep 6;223(5210):1022–1024. doi: 10.1038/2231022a0. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- COLBOURN J. L., WITHERSPOON B. H., HERBST E. J. Effect of intracellular spermine on ribosomes of Escherichia coli. Biochim Biophys Acta. 1961 May 13;49:422–424. doi: 10.1016/0006-3002(61)90155-x. [DOI] [PubMed] [Google Scholar]
- Chambon P., Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. X. Ribosomes and nucleic acids of vegetative cells and spores of Bacillus megaterium. J Biol Chem. 1968 Oct 10;243(19):5110–5116. [PubMed] [Google Scholar]
- DOI R. H., IGARASHI R. T. GENETIC TRANSCRIPTION DURING MORPHOGENESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:755–762. doi: 10.1073/pnas.52.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., NIEDERPRUEM D. J. ELECTRON TRANSPORT SYSTEM IN VEGETATIVE CELLS AND MICROCYSTS OF MYXOCOCCUS XANTHUS. J Bacteriol. 1964 Feb;87:316–322. doi: 10.1128/jb.87.2.316-322.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. Biology of the myxobacteria. Annu Rev Microbiol. 1966;20:75–106. doi: 10.1146/annurev.mi.20.100166.000451. [DOI] [PubMed] [Google Scholar]
- Dworkin M., Sadler W. Induction of cellular morphogenesis in Myxococcus xanthus. I. General description. J Bacteriol. 1966 Apr;91(4):1516–1519. doi: 10.1128/jb.91.4.1516-1519.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Kennel D. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J Mol Biol. 1968 May 28;34(1):85–103. doi: 10.1016/0022-2836(68)90236-2. [DOI] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Titration of the gene sites on DNA by DNA-RNA hybridization. I. Problem of measurement. J Mol Biol. 1968 May 28;34(1):71–84. doi: 10.1016/0022-2836(68)90235-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Maitra U., Barash F. DNA-dependent synthesis of RNA by Escherichia coli RNA polymerase: release and reinitiation of RNA chains from DNA templates. Proc Natl Acad Sci U S A. 1969 Oct;64(2):779–786. doi: 10.1073/pnas.64.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Ames B. N. THE EFFECT OF POLYAMINES AND OF POLY U SIZE ON PHENYLALANINE INCORPORATION. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2171–2178. doi: 10.1073/pnas.48.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. W., Erdmann V. A., Herbst E. J. Polyamine-inorganic cation interaction with ribosomes of Escherichia coli. Biochim Biophys Acta. 1968 Jan 29;155(1):293–295. doi: 10.1016/0005-2787(68)90359-6. [DOI] [PubMed] [Google Scholar]
- Ramsey W. S., Dworkin M. Stable messenger ribonucleic acid and germination of Myxococcus xanthus microcysts. J Bacteriol. 1970 Feb;101(2):531–540. doi: 10.1128/jb.101.2.531-540.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Katarski M., Gottlieb P. Deoxyribonucleic acid synthesis during exponential growth and microcyst formation in Myxococcus xanthus. J Bacteriol. 1967 Apr;93(4):1402–1408. doi: 10.1128/jb.93.4.1402-1408.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler W., Dworkin M. Induction of cellular morphogenesis in Myxococcus xanthus. II. Macromolecular synthesis and mechanism of inducer action. J Bacteriol. 1966 Apr;91(4):1520–1525. doi: 10.1128/jb.91.4.1520-1525.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silman N., Artman M., Engleberg H. Effect of magnesium and spermine on the aggregation of bacterial and mammalian ribosomes. Biochim Biophys Acta. 1965 Jun 8;103(2):231–240. doi: 10.1016/0005-2787(65)90164-4. [DOI] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Bock R. M. Isolation and physical properties of the ribosomal ribonucleic acid of Escherichia coli. Biochemistry. 1965 Jul;4(7):1302–1311. doi: 10.1021/bi00883a014. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Okamoto T., Takanami M. RNA polymerase sigma-factor and the selection of initiation site. Nature. 1970 Feb 14;225(5233):598–600. doi: 10.1038/225598a0. [DOI] [PubMed] [Google Scholar]
- Sussman R. R. RNA metabolism during cytodifferentiation in the cellular slime mold Polysphondelium pallidum. Biochim Biophys Acta. 1967 Dec 19;149(2):407–421. doi: 10.1016/0005-2787(67)90169-4. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Burgessrr Cyclic re-use of the RNA polymerase sigma factor. Nature. 1969 May 10;222(5193):537–540. doi: 10.1038/222537a0. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Mizuno S., Yamazaki H., Nitta K. Inhibition of DNA-dependent RNA synthesis by rifamycins. J Antibiot (Tokyo) 1968 Mar;21(3):234–236. doi: 10.7164/antibiotics.21.234. [DOI] [PubMed] [Google Scholar]
- VOELZ H., DWORKIN M. Fine structure of Myxococcus xanthus during morphogenesis. J Bacteriol. 1962 Nov;84:943–952. doi: 10.1128/jb.84.5.943-952.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B. F., Dworkin M. Comparative intermediary metabolism of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1968 Nov;96(5):1465–1473. doi: 10.1128/jb.96.5.1465-1473.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., Dworkin M., Tipper D. J. Peptidoglycan of Myxococcus xanthus: structure and relation to morphogenesis. J Bacteriol. 1968 Jun;95(6):2186–2197. doi: 10.1128/jb.95.6.2186-2197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Rosenberg E. Induction of morphogenesis by methionine starvation in Myxococcus xanthus: polyamine control. J Bacteriol. 1970 Sep;103(3):641–649. doi: 10.1128/jb.103.3.641-649.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Chemical and morphological studies of bacterial spore formation. II. Spore and parasporal protein formation in Bacillus cereus var. alesti. J Biophys Biochem Cytol. 1959 Dec;6:483–498. doi: 10.1083/jcb.6.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]


