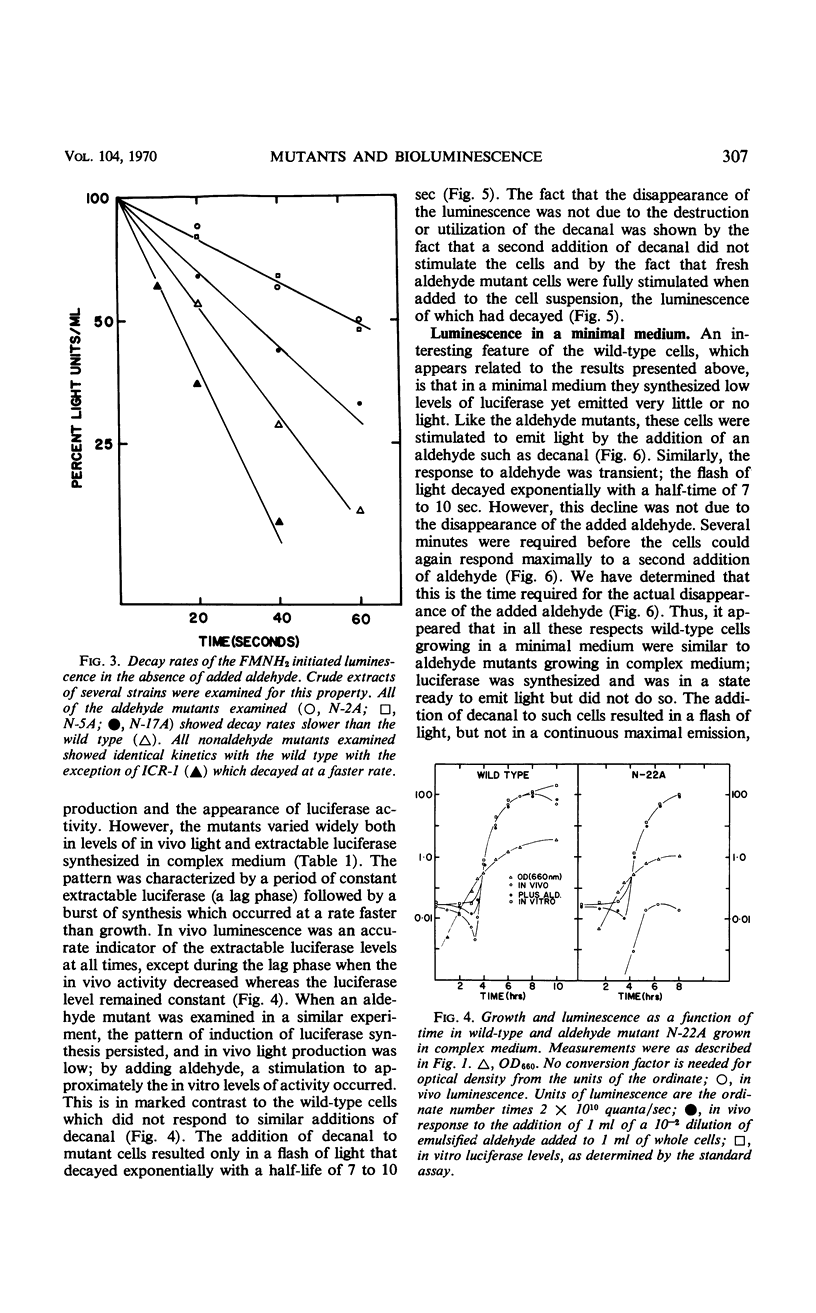
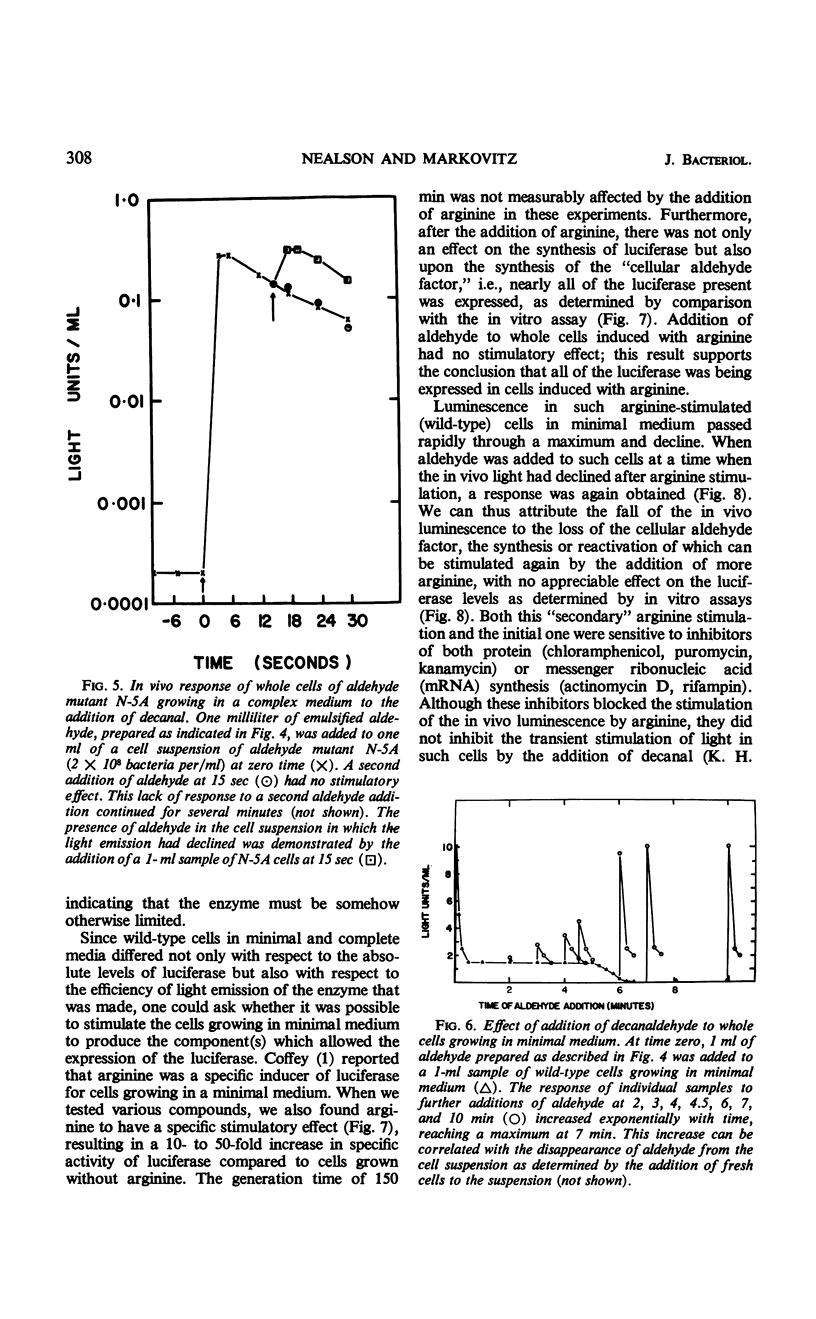
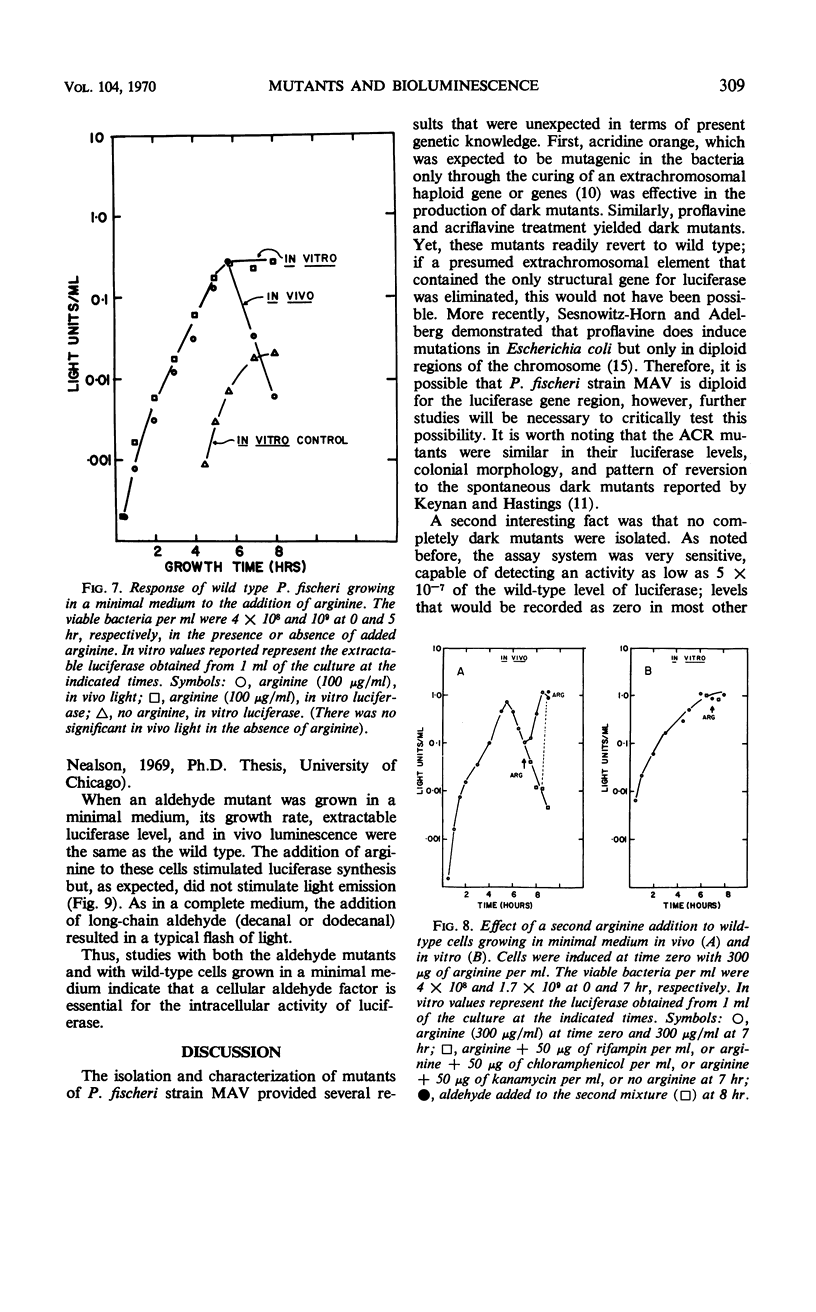
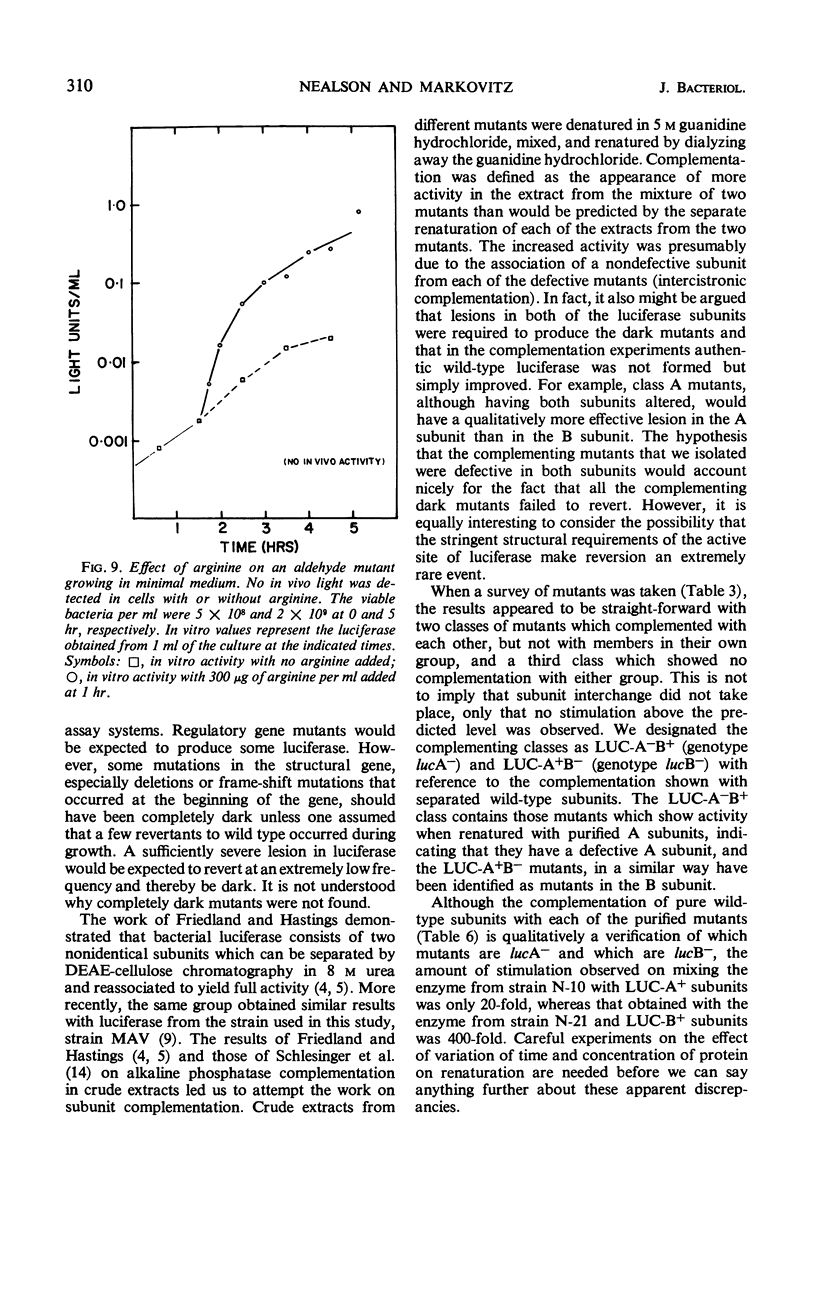
Abstract
Chemical mutagens were used to obtain mutants deficient in bioluminescence in the marine bacterium Photobacterium fischeri strain MAV. Acridine dyes were effective in the production of dark mutants but not in the production of auxotrophs. These dark mutants were all of one type and appeared to contain lesions blocking the synthesis of luciferase. ICR-191 was especially effective in the production of aldehyde mutants, i.e., dark strains that luminesce when a long-chain aldehyde such as n-decanal is added to them. However, other mutant types were isolated after treatment with ICR-191. N-methyl-N′-nitro-N-nitrosoguanidine induced many bioluminescence-deficient types with respect to both the site of the lesion and the quantitative effect on the luminescent system. We characterized the dark and dim mutants with respect to their response to exogenous decanal, levels of in vivo and in vitro luminescence, and their rates of reversion to wild type. In addition, the luciferases of the mutant strains were examined by subunit complementation. On the basis of these analyses, we identified mutants which synthesize altered luciferase, strains which are deficient in synthesis of luciferase, and aldehyde mutants. The results of analysis of luciferase from the aldehyde mutants and the complementation studies indicate that the lesions in these strains are in the luciferase itself. Results obtained with wild-type cells grown in minimal medium, and aldehyde mutant cells grown either in complete or minimal medium, indicate that a “natural aldehyde factor” is involved in in vivo light emission. These same studies showed that the long-chain aldehyde(s) could only partially substitute for the natural “aldehyde factor.” The possibility that the in vivo aldehyde factor is not a long-chain aldehyde is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffey J. J. Inducible synthesis of bacterial luciferase: specificity and kinetics of induction. J Bacteriol. 1967 Nov;94(5):1638–1647. doi: 10.1128/jb.94.5.1638-1647.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGHALY A. H. Factors influencing the growth and light production of luminous bacteria. J Cell Physiol. 1950 Oct;36(2):165–183. doi: 10.1002/jcp.1030360205. [DOI] [PubMed] [Google Scholar]
- Friedland J., Hastings J. W. Nonidentical subunits of bacterial luciferase: their isolation and recombination to form active enzyme. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2336–2342. doi: 10.1073/pnas.58.6.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J., Hastings J. W. The reversibility of the denaturation of bacterial luciferase. Biochemistry. 1967 Sep;6(9):2893–2900. doi: 10.1021/bi00861a033. [DOI] [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H. Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J Biol Chem. 1963 Jul;238:2537–2554. [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- Hastings J. W., Weber K., Friedland J., Eberhard A., Mitchell G. W., Gunsalus A. Structurally distinct bacterial luciferases. Biochemistry. 1969 Dec;8(12):4681–4689. doi: 10.1021/bi00840a004. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G., Hastings J. W. The effect of flavin isomers and analogues upon the color of bacterial bioluminescence. J Biol Chem. 1969 May 25;244(10):2572–2576. [PubMed] [Google Scholar]
- Rogers P., McElroy W. D. BIOCHEMICAL CHARACTERISTICS OF ALDEHYDE AND LUCIFERASE MUTANTS OF LUMINOUS BACTERIA. Proc Natl Acad Sci U S A. 1955 Feb 15;41(2):67–70. doi: 10.1073/pnas.41.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREHLER B. L., CORMIER M. J. Isolation, identification, and function of long chain fatty aldehydes affecting the bacterial luciferin-luciferase reaction. J Biol Chem. 1954 Nov;211(1):213–225. [PubMed] [Google Scholar]
- Sesnowitz-Horn S., Adelberg E. A. Proflavin-induced mutations in the L-arabinose operon of Escherichia coli. I. Production and genetic analyses of such mutations. J Mol Biol. 1969 Nov 28;46(1):1–15. doi: 10.1016/0022-2836(69)90053-9. [DOI] [PubMed] [Google Scholar]


