Abstract
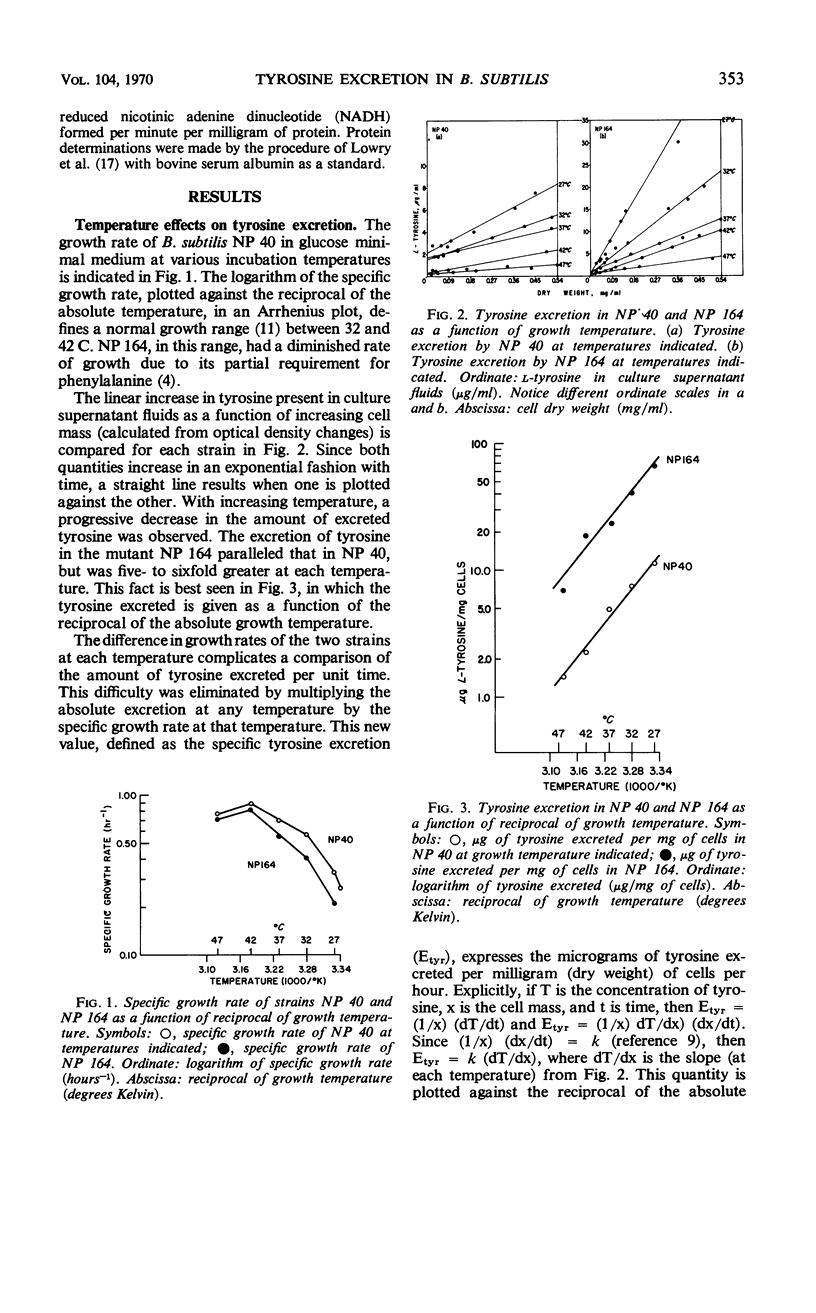
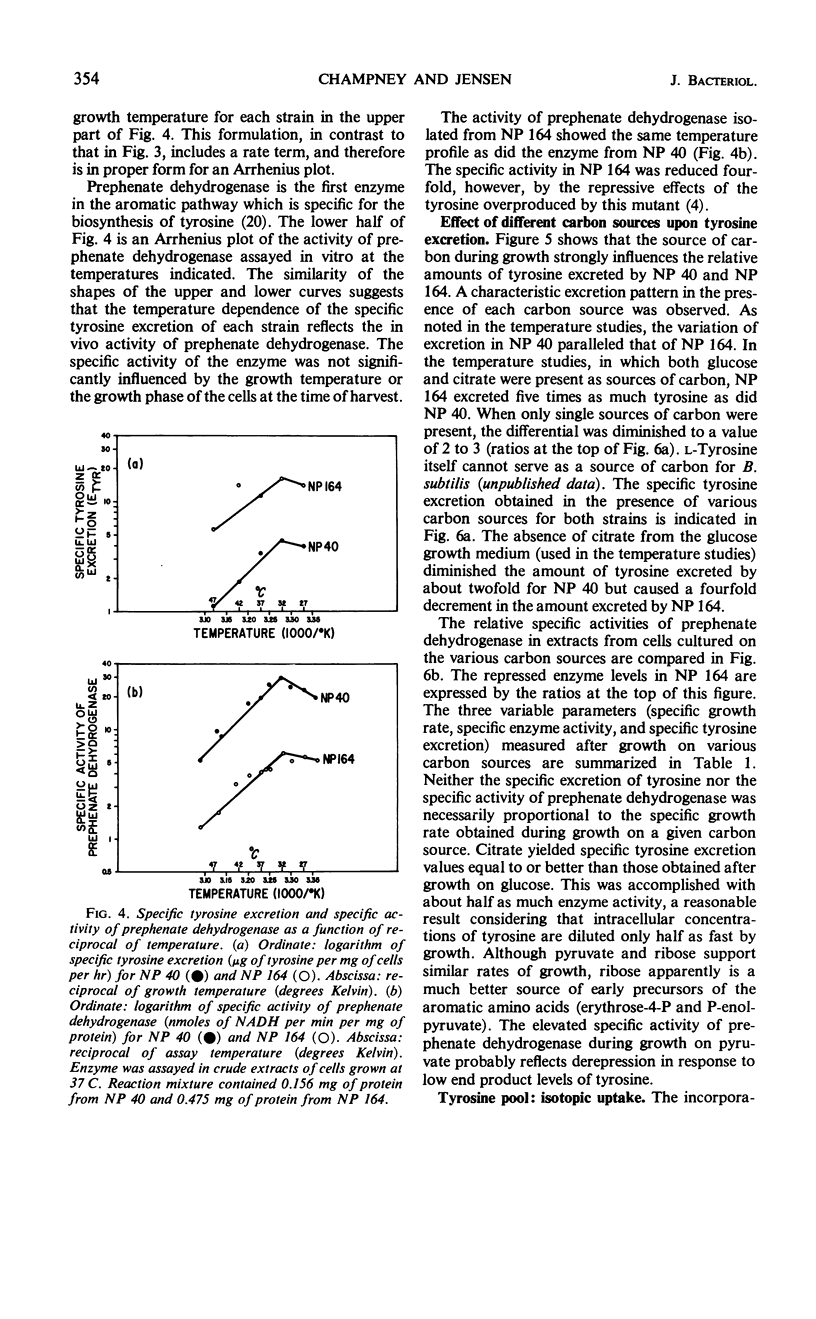
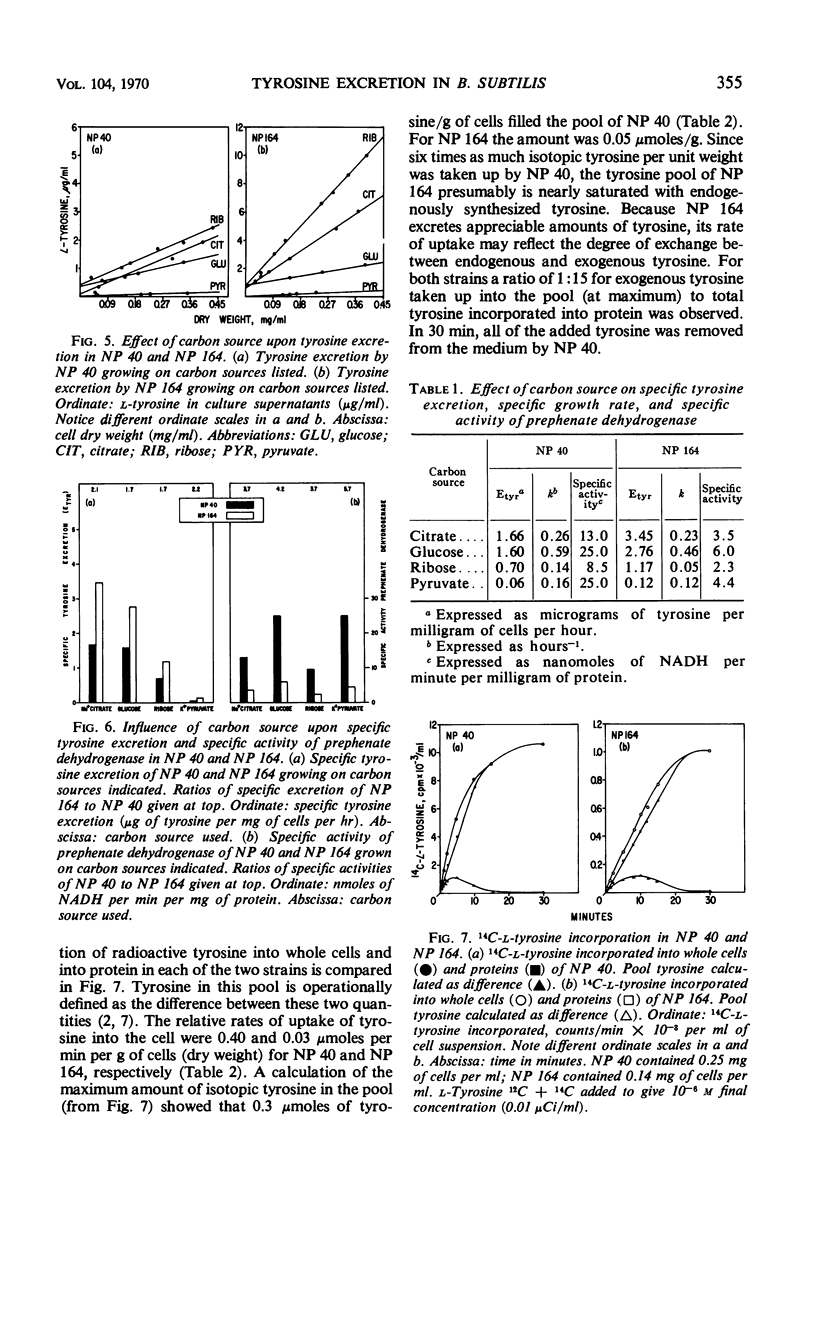
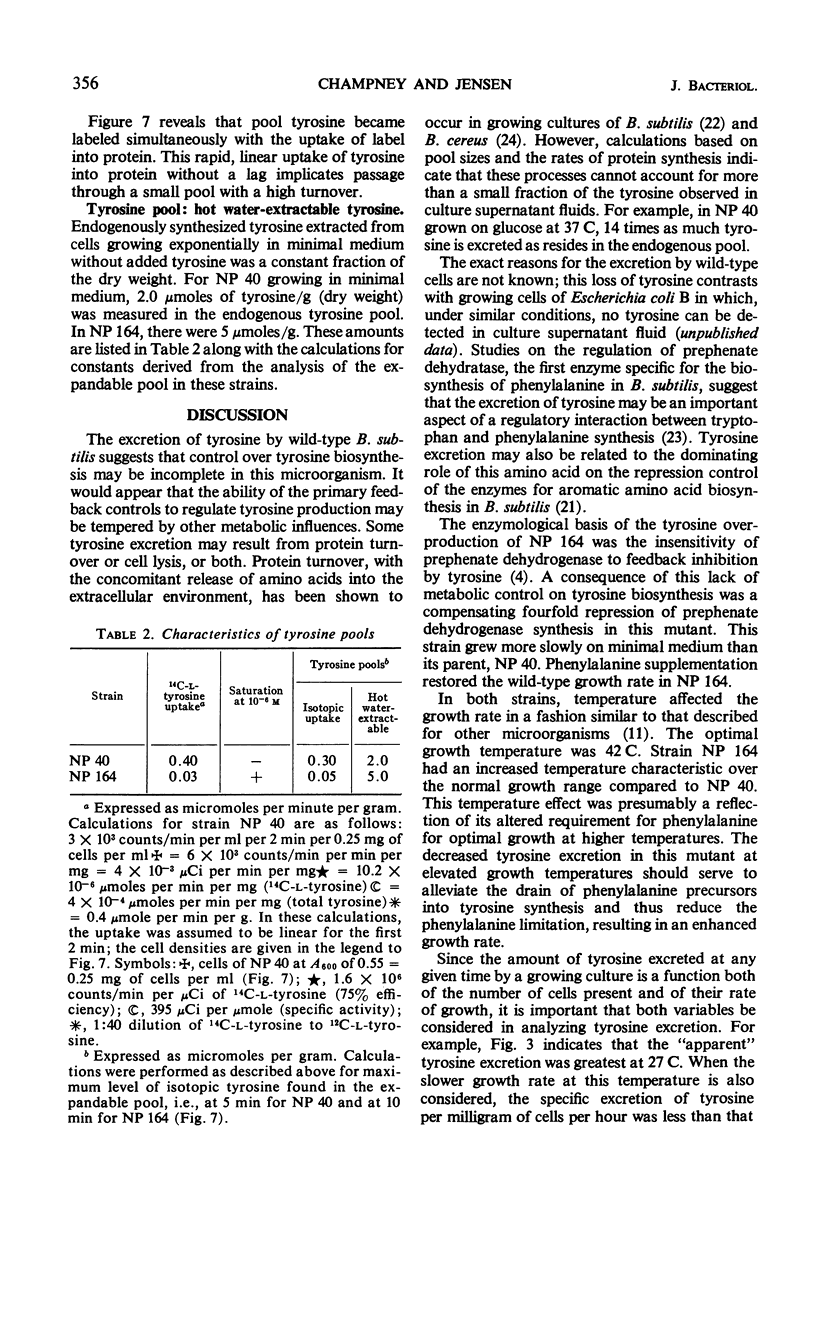
The biosynthetic pathway for tyrosine synthesis is regulated both by repression of enzyme synthesis and by feedback inhibition of enzyme activity in Bacillus subtilis. Nevertheless, wild-type cells produce significantly more tyrosine than is required for protein synthesis, and part of this is excreted into the medium. Alteration of nutritional and other environmental conditions of cultivation strongly influenced the amount of tyrosine excretion. The excretion of tyrosine by wild-type cells was compared with that of a regulatory mutant having a feedback-insensitive prephenate dehydrogenase. Tyrosine excretion varied directly with the in vitro activity of prephenate dehydrogenase and inversely with temperature in the two strains. The regulatory mutant excreted five times as much tyrosine as the wild type at all growth temperatures examined. The carbon source used for growth significantly influenced the level of tyrosine excretion. The specific activity of prephenate dehydrogenase was also affected by the carbon source. Incorporation studies with isotopic tyrosine and fluorometric determinations of tyrosine concentrations extractable in hot water were used to measure operationally the tyrosine pools in the mutant and wild-type strains. The effects of various environmental conditions on the synthesis and excretion of tyrosine led to the conclusion that metabolic controls governing end product contrations exist which are completely independent of regulation by feedback inhibition and repression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAO K. C., FOSTER J. W. A glutamic acid-producing bacillus. J Bacteriol. 1959 Jun;77(6):715–725. doi: 10.1128/jb.77.6.715-725.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWIE D. B., McCLURE F. T. Metabolic pools and the synthesis of macromolecules. Biochim Biophys Acta. 1959 Jan;31(1):236–245. doi: 10.1016/0006-3002(59)90460-3. [DOI] [PubMed] [Google Scholar]
- Champney W. S., Jensen R. A. D-Tyrosine as a metabolic inhibitor of Bacillus subtilis. J Bacteriol. 1969 Apr;98(1):205–214. doi: 10.1128/jb.98.1.205-214.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin L. A., Magasanik B. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J Biol Chem. 1968 Oct 10;243(19):5165–5178. [PubMed] [Google Scholar]
- Demain A. L. Industrial fermentations and their relation to regulatory mechanisms. Adv Appl Microbiol. 1966;8:1–27. doi: 10.1016/s0065-2164(08)70490-8. [DOI] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., Neidhardt F. C. Effect of growth rate on histidine catabolism and histidase synthesis in Aerobacter aerogenes. J Bacteriol. 1969 Apr;98(1):131–142. doi: 10.1128/jb.98.1.131-142.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. A biochemical basis for apparent abortive transformation in Bacillus subtilis. Genetics. 1968 Dec;60(4):707–717. doi: 10.1093/genetics/60.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINOSHITA S. The production of amino acids by fermentation processes. Adv Appl Microbiol. 1959;1:201–214. doi: 10.1016/s0065-2164(08)70480-5. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid pool formation in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):282–291. doi: 10.1128/jb.97.1.282-291.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):273–281. doi: 10.1128/jb.97.1.273-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A., Nasser D. S. Regulation of enzyme synthesis in the aromatic amino acid pathway of Bacillus subtilus. J Bacteriol. 1969 Jan;97(1):83–90. doi: 10.1128/jb.97.1.83-90.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello J. L., Jensen R. A. Metabolic interlock. The multi-metabolite control of prephenate dehydratase activity in Bacillus subtilis. J Biol Chem. 1970 Aug 10;245(15):3738–3744. [PubMed] [Google Scholar]
- URBA R. C. Protein breakdown in Bacillus cereus. Biochem J. 1959 Mar;71(3):513–518. doi: 10.1042/bj0710513. [DOI] [PMC free article] [PubMed] [Google Scholar]



