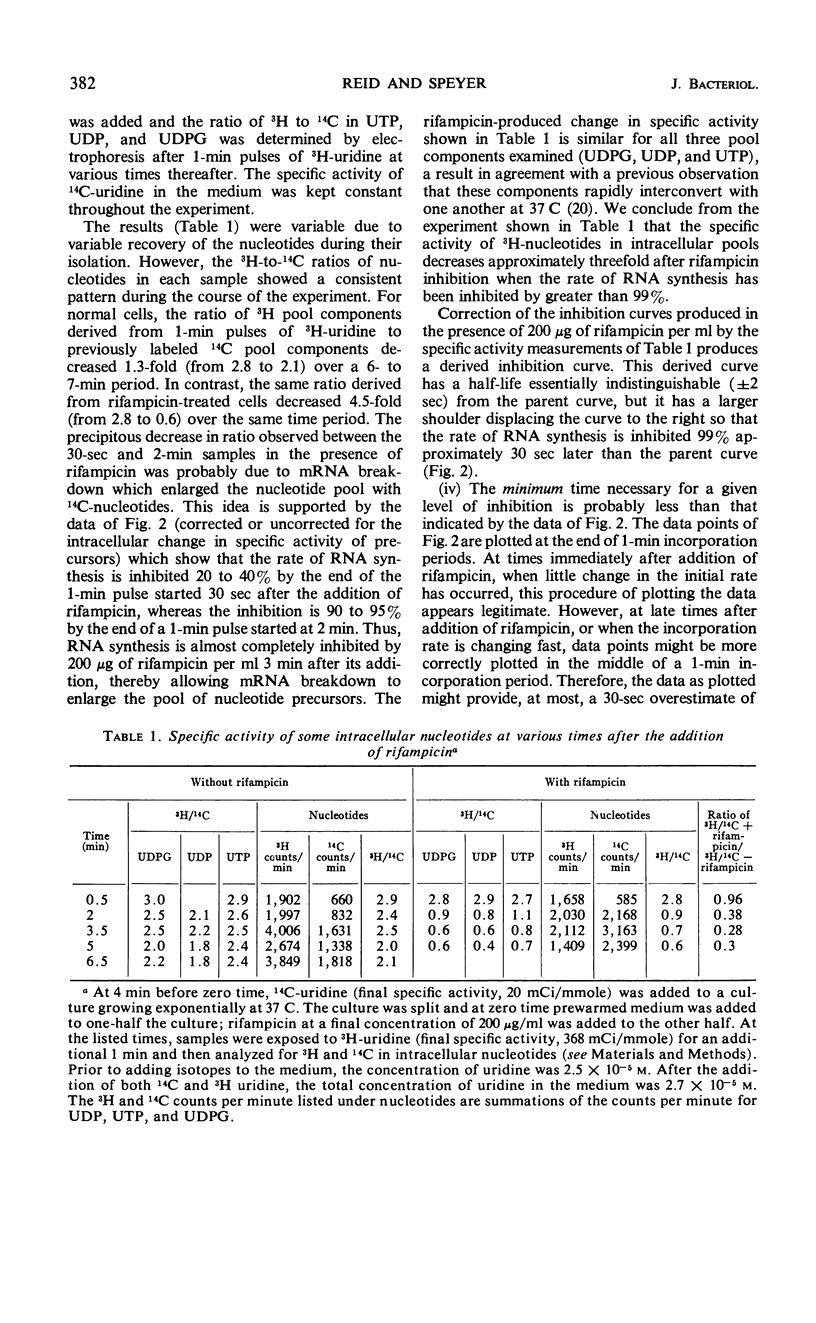
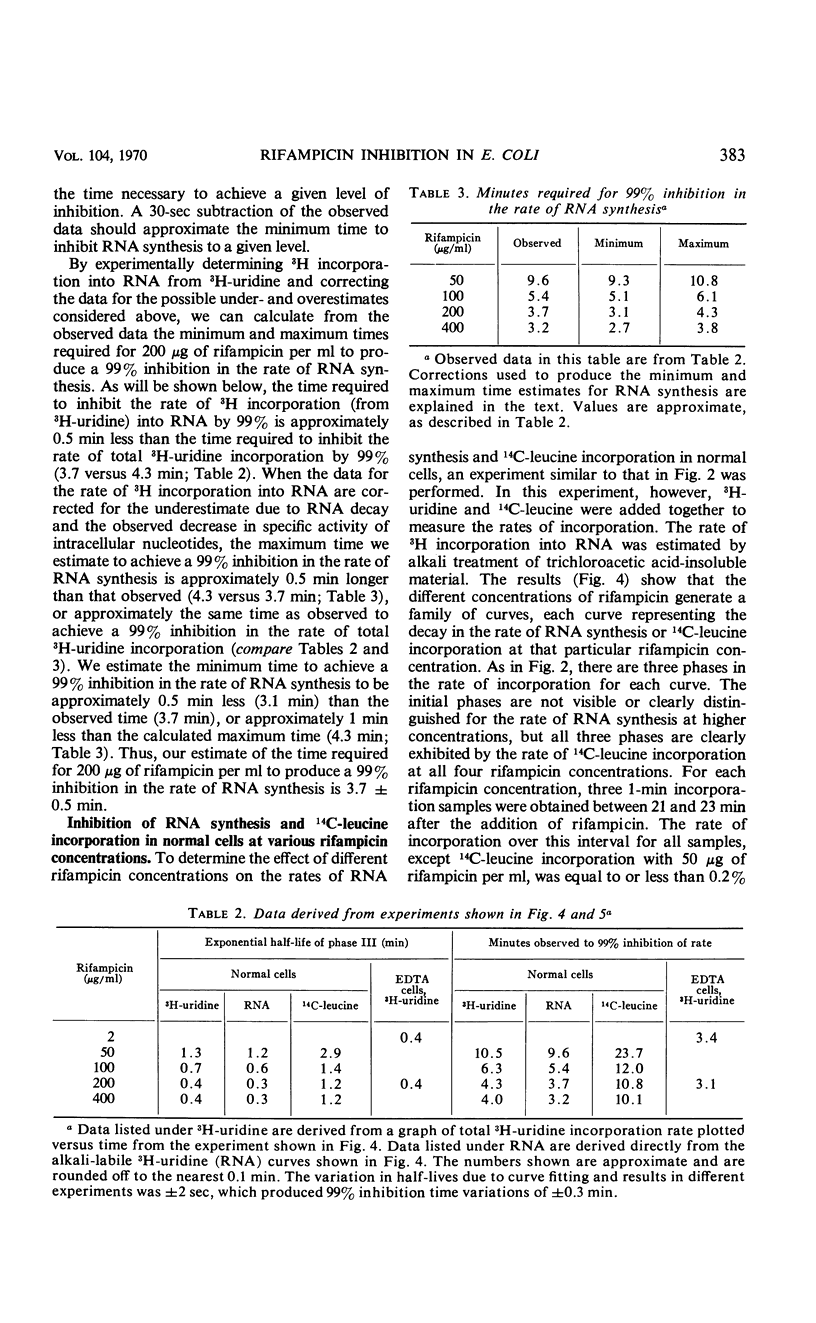
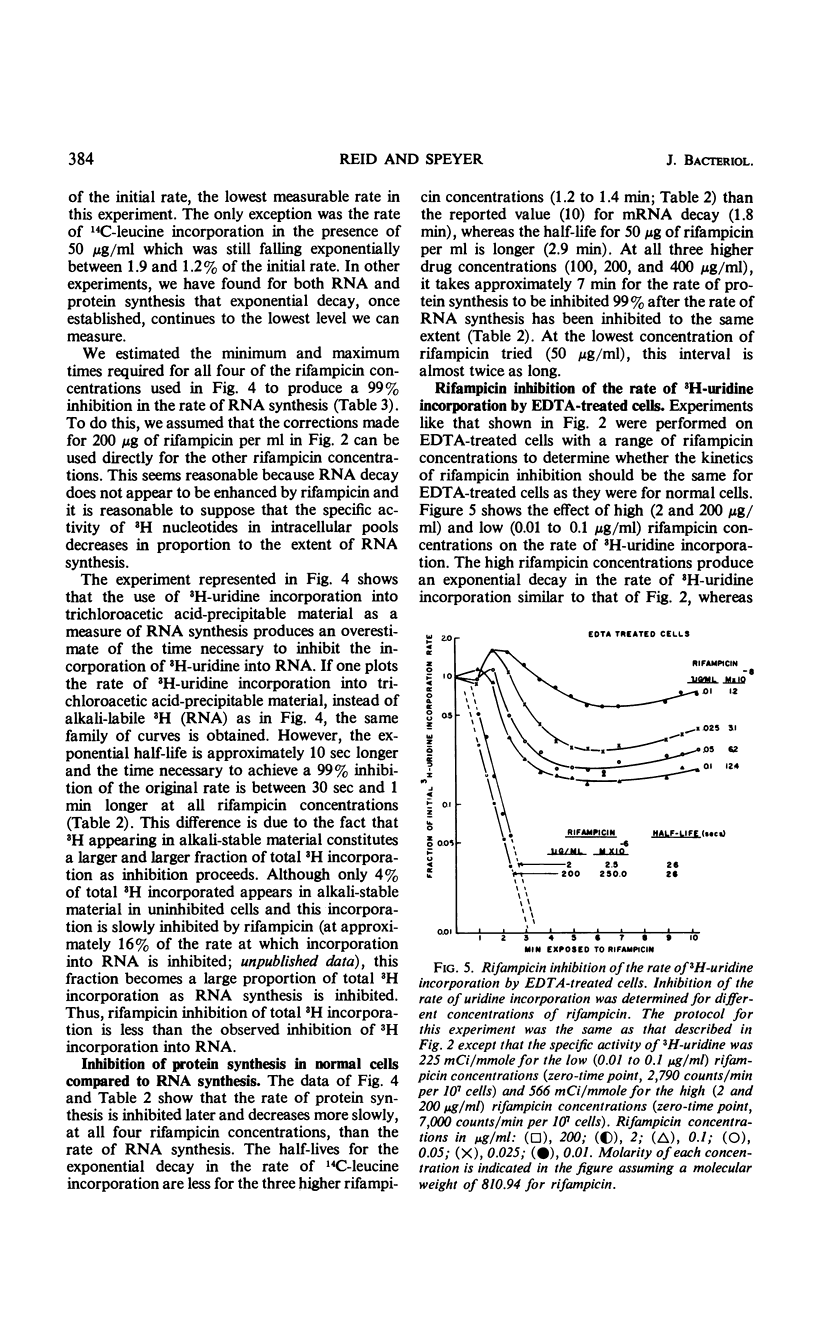
Abstract
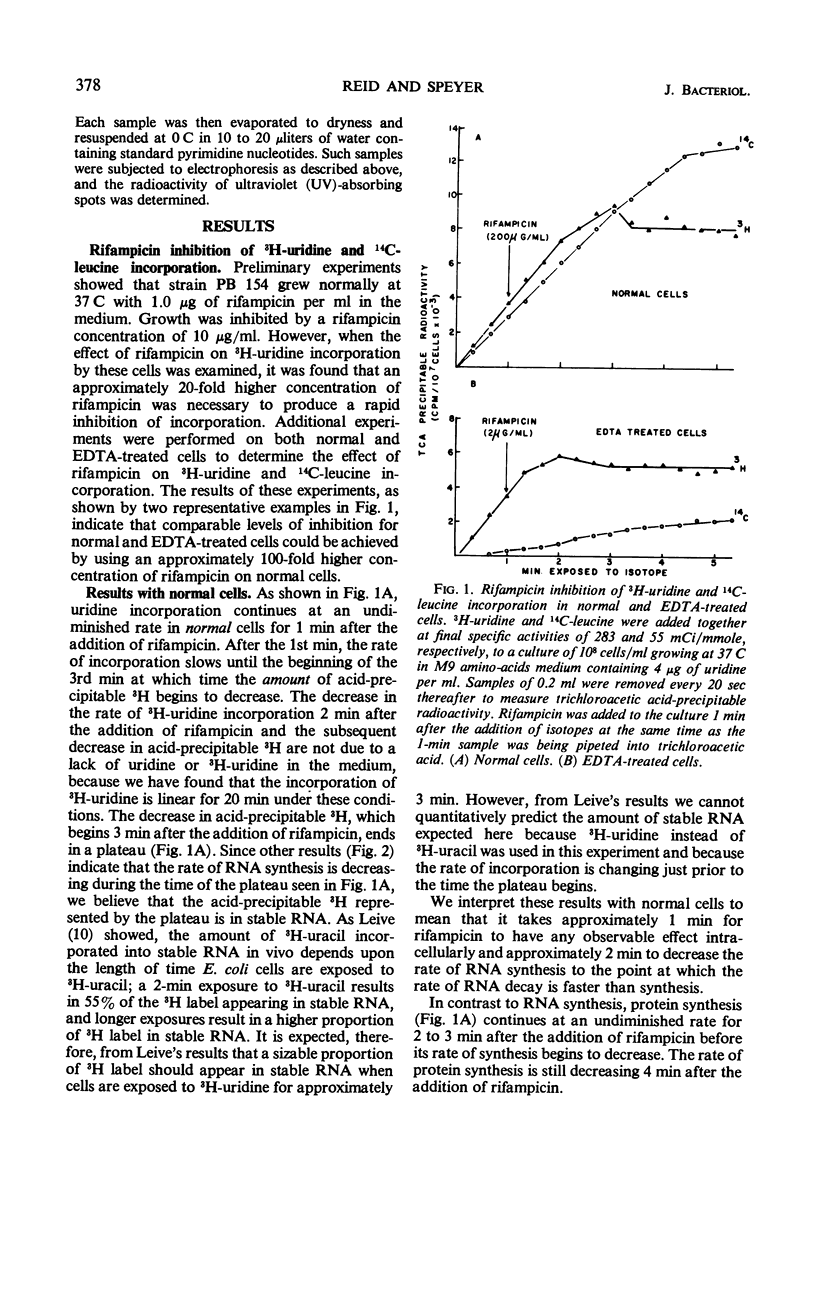
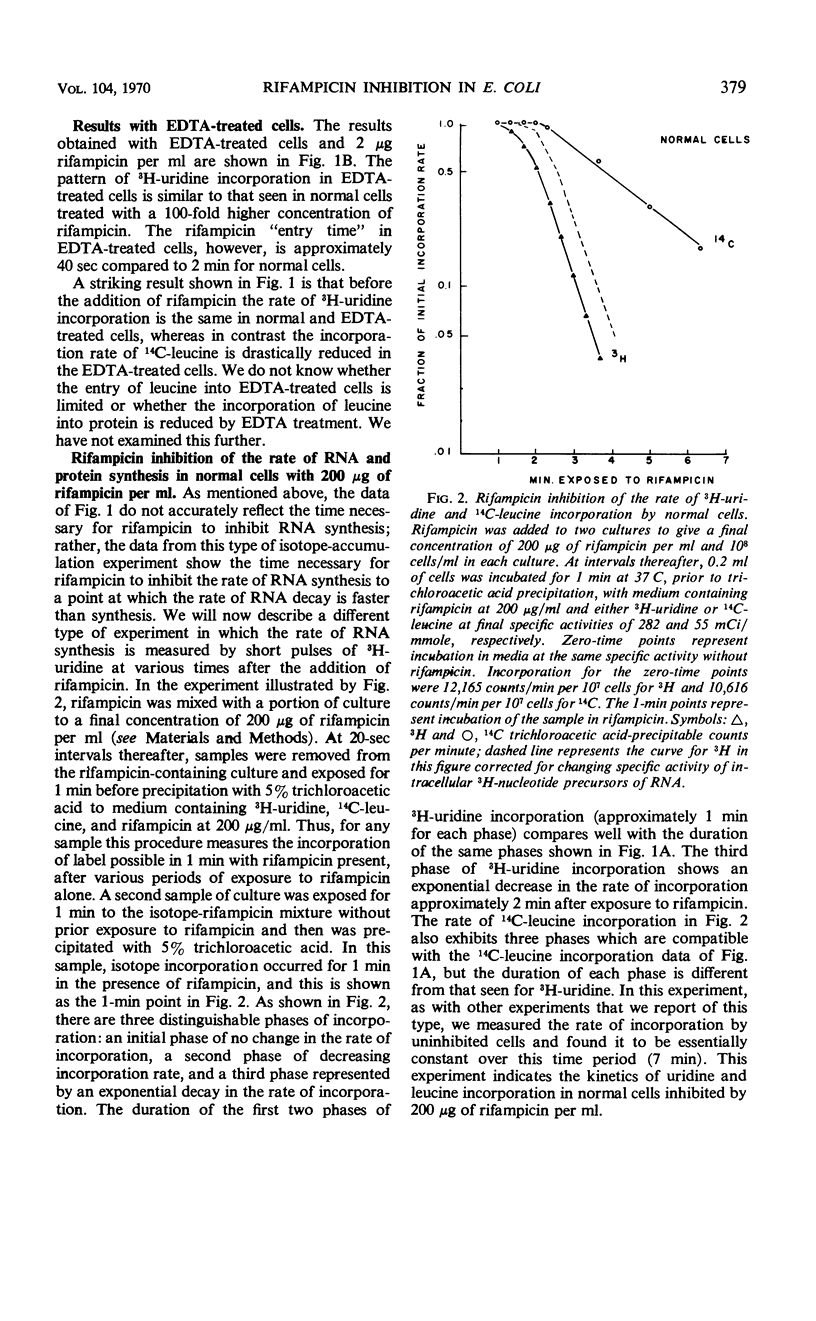
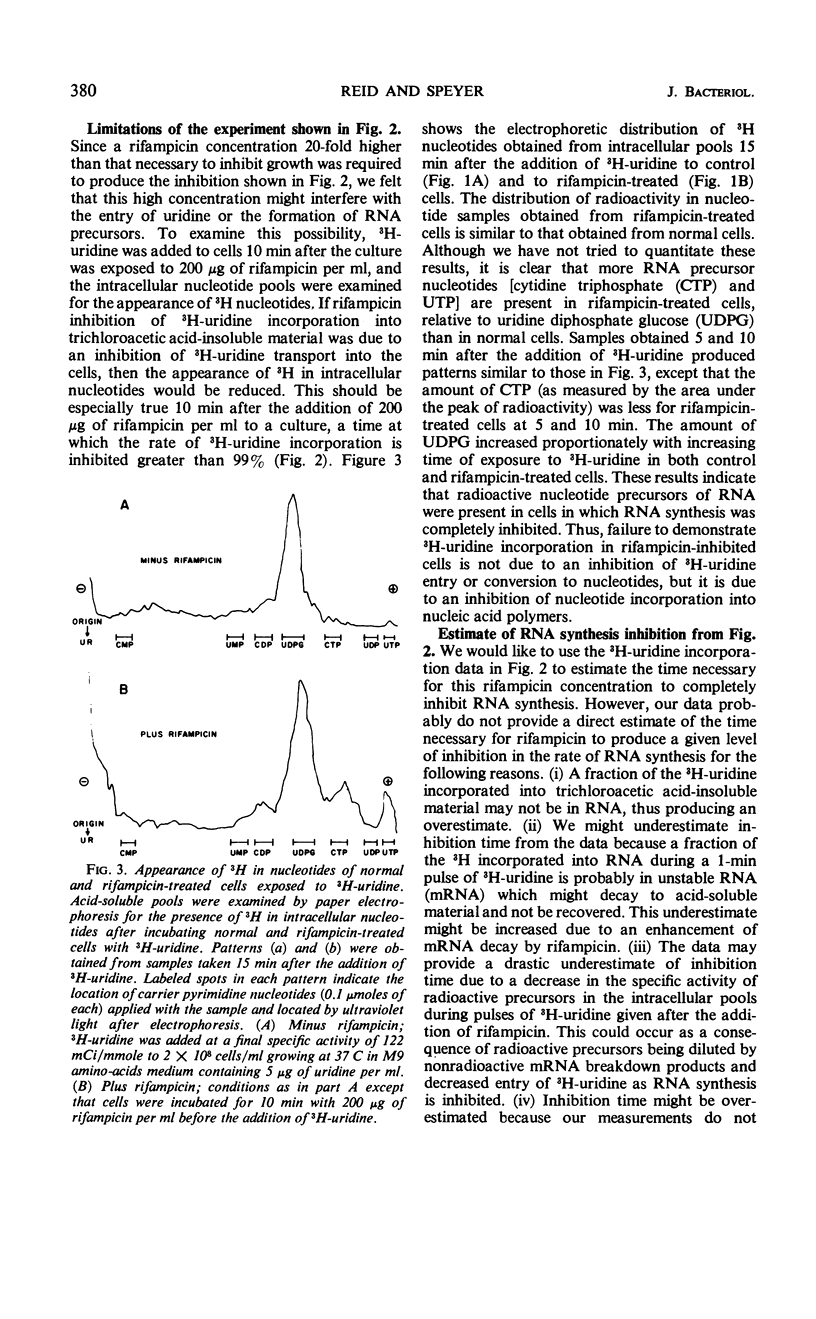
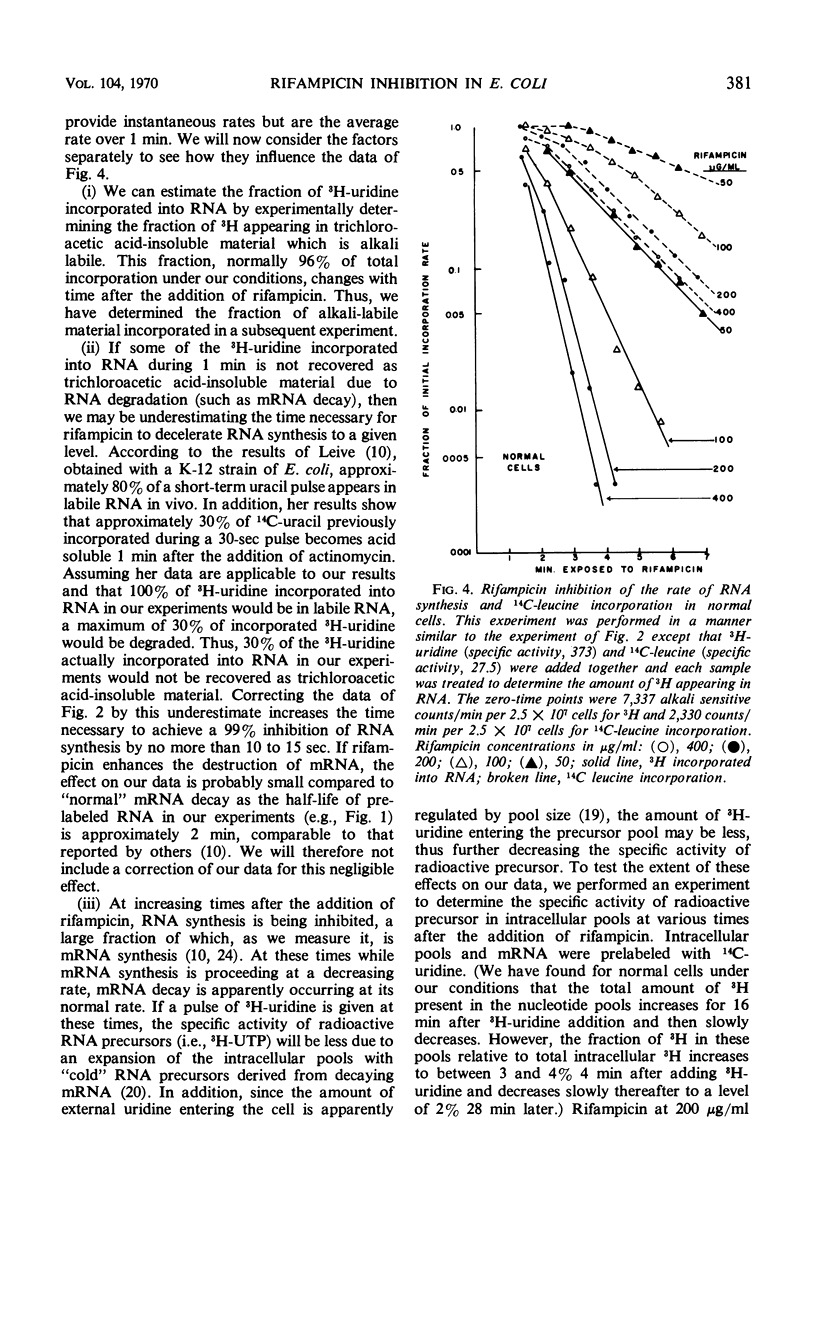
The kinetics of ribonucleic acid (RNA) and protein synthesis in rifampicin-inhibited normal and ethylenediaminetetraacetic acid (EDTA)-treated Escherichia coli was measured. Approximately 200-fold higher external concentrations of rifampicin were needed to produce a level of inhibition in normal cells comparable to that observed in EDTA-treated cells. The rates of RNA and protein synthesis in both kinds of cells decreased exponentially, after an initial lag phase, at all rifampicin concentrations tested. The lag phase was longer and the final exponential slope less for protein synthesis than for RNA synthesis at a given rifampicin concentration. Below certain rifampicin concentrations, both the lag phase and the subsequent exponential decrease in the rates of RNA and protein synthesis were found to be rifampicin concentration dependent. At greater concentrations only the time of the lag phase was decreased by higher rifampicin concentrations, whereas the slope of the exponential decrease in the rates of RNA and protein synthesis was unaffected. In all cases, the exponential decrease continued to at least a 99.8% inhibition of the original rate of synthesis. These in vivo results are consistent with the mode of rifampicin action determined from in vitro studies; rifampicin prevents initiations of RNA polymerase on deoxyribonucleic acid, but not its propagation, by binding the enzyme essentially irreversibly. The results also indicate the size distribution of messenger RNA molecules in E. coli under our conditions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Levinthal C. Synthesis and maturation of ribosomal RNA in Escherichia coli. J Mol Biol. 1969 Dec 14;46(2):281–303. doi: 10.1016/0022-2836(69)90422-7. [DOI] [PubMed] [Google Scholar]
- BENZINGER R., HARTMAN P. E. Effects of ultraviolet light on transducing phage P22. Virology. 1962 Dec;18:614–626. doi: 10.1016/0042-6822(62)90064-8. [DOI] [PubMed] [Google Scholar]
- Bremer H., Yuan D. Chain growth rate of messenger RNA in Escherichia coli infected with bacteriophage T4. J Mol Biol. 1968 Jun 28;34(3):527–540. doi: 10.1016/0022-2836(68)90178-2. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Vogel M., Brown R. D. Conservation of the rifamycin sensitivity of transcription during T4 development. Nature. 1969 Mar 1;221(5183):836–838. doi: 10.1038/221836a0. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Keberle H., Meyer-Brunot H., Schmid K. Pharmacokinetic and metabolic studies with labeled rifamycin antibiotics. Antimicrob Agents Chemother (Bethesda) 1966;6:365–370. [PubMed] [Google Scholar]
- Lancini G. C., Sartori G. Rifamycins LXI: in vivo inhibition of RNA synthesis of rifamycins. Experientia. 1968 Nov 15;24(11):1105–1106. doi: 10.1007/BF02147783. [DOI] [PubMed] [Google Scholar]
- Maggi N., Pasqualucci C. R., Ballotta R., Sensi P. Rifampicin: a new orally active rifamycin. Chemotherapy. 1966;11(5):285–292. doi: 10.1159/000220462. [DOI] [PubMed] [Google Scholar]
- Maitra U., Barash F. DNA-dependent synthesis of RNA by Escherichia coli RNA polymerase: release and reinitiation of RNA chains from DNA templates. Proc Natl Acad Sci U S A. 1969 Oct;64(2):779–786. doi: 10.1073/pnas.64.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Nakata Y., Hurwitz J. The role of deoxyribonucleic acid in ribonucleic acid synthesis. XIV. A study of the initiation of ribonucleic acid synthesis. J Biol Chem. 1967 Nov 10;242(21):4908–4918. [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- Marino P., Baldi M. I., Tocchini-Valentini G. P. Effect of rifampicin on DNA-dependent RNA polymerase and on RNA phage growth. Cold Spring Harb Symp Quant Biol. 1968;33:125–127. doi: 10.1101/sqb.1968.033.01.016. [DOI] [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutant of bacteriophage T4. I. Ribonucleic acid metabolism. J Biol Chem. 1968 Nov 10;243(21):5610–5615. [PubMed] [Google Scholar]
- Morse D. E., Baker R. F., Yanofsky C. Translation of the tryptophan messenger RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1428–1435. doi: 10.1073/pnas.60.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Transcription of the tryptophan operon in Escherichia coli: rifampicin as an inhibitor of initiation. J Mol Biol. 1970 Mar;48(3):525–531. doi: 10.1016/0022-2836(70)90064-1. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Radioisotope uptake as a measure of synthesis of messenger RNA. Science. 1967 Dec 1;158(3805):1186–1188. doi: 10.1126/science.158.3805.1186. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P., Vielmetter W. Kinetic studies on the relationship of ribonucleotide precursor pools and ribonucleic acid synthesis. J Mol Biol. 1968 Feb 28;32(1):135–147. doi: 10.1016/0022-2836(68)90151-4. [DOI] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Matsson E. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J Bacteriol. 1969 Mar;97(3):1334–1342. doi: 10.1128/jb.97.3.1334-1342.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson A. E., Pispa J. P., Buchanan J. M. Transient activation of RNA polymerase in Escherichia coli B after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1969 Jun;63(2):473–480. doi: 10.1073/pnas.63.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Isolation and characterization of streptolydigin resistant RNA polymerase. Nature. 1969 Sep 6;223(5210):1068–1069. doi: 10.1038/2231068a0. [DOI] [PubMed] [Google Scholar]
- Sedat J., Lyon A., Sinsheimer R. L. Purification of Escherichia coli pulse-labeled RNA by benzoylated DEAE-cellulose chromatography. J Mol Biol. 1969 Sep 28;44(3):415–434. doi: 10.1016/0022-2836(69)90370-2. [DOI] [PubMed] [Google Scholar]
- Sensi P., Maggi N., Füresz S., Maffii G. Chemical modifications and biological properties of rifamycins. Antimicrob Agents Chemother (Bethesda) 1966;6:699–714. [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Control of template specificity of E. coli RNA polymerase by a phage-coded protein. Nature. 1969 Sep 13;223(5211):1111–1113. doi: 10.1038/2231111a0. [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Marino P., Colvill A. J. Mutant of E. coli containing an altered DNA-dependent RNA polymerase. Nature. 1968 Oct 19;220(5164):275–276. doi: 10.1038/220275a0. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Mizuno S., Yamazaki H., Nitta K. Inhibition of DNA-dependent RNA synthesis by rifamycins. J Antibiot (Tokyo) 1968 Mar;21(3):234–236. doi: 10.7164/antibiotics.21.234. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. The rifamycins--relation of chemical structure and action on RNA polymerase. Biochim Biophys Acta. 1969 May 20;182(1):24–29. doi: 10.1016/0005-2787(69)90516-4. [DOI] [PubMed] [Google Scholar]


