Abstract
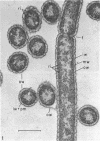
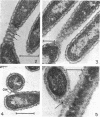
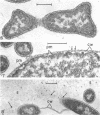
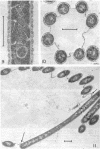
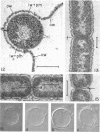
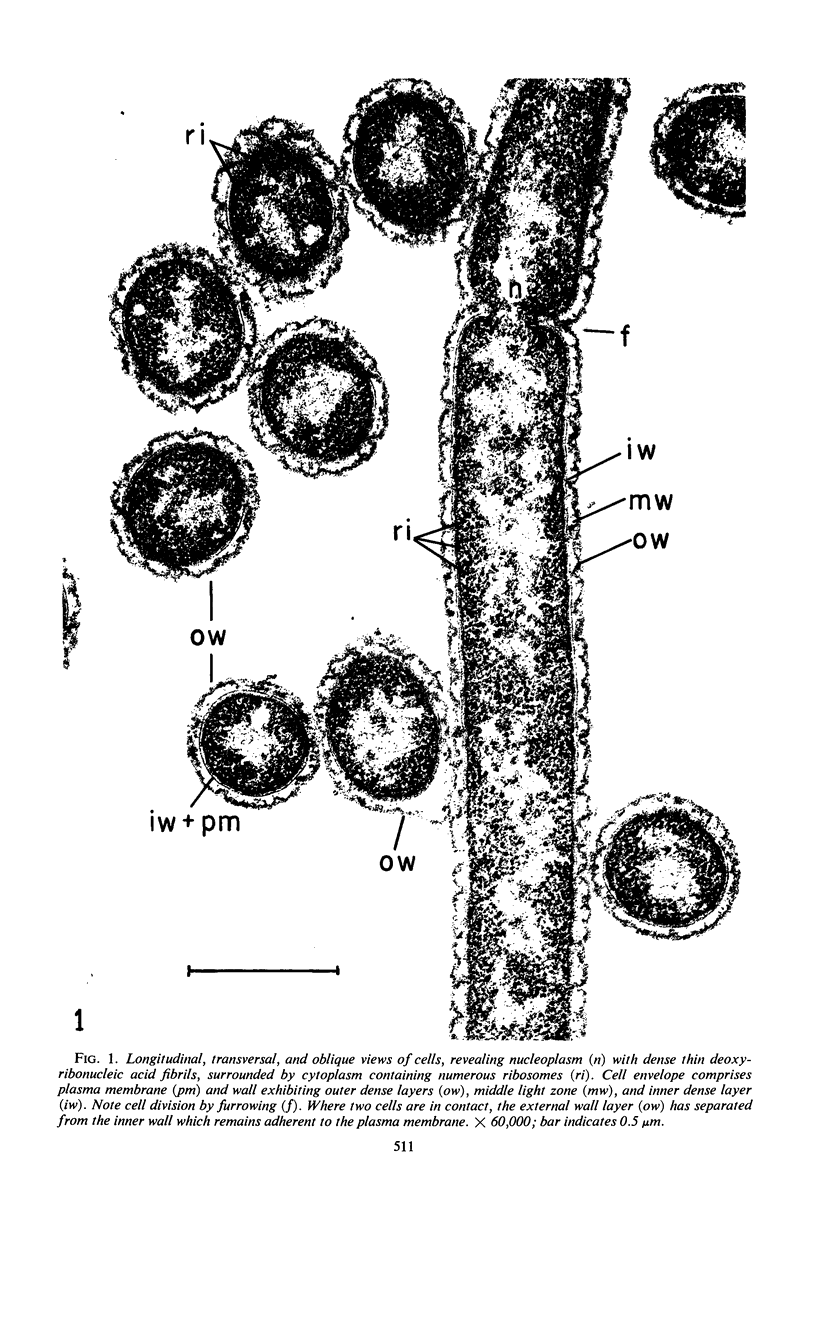
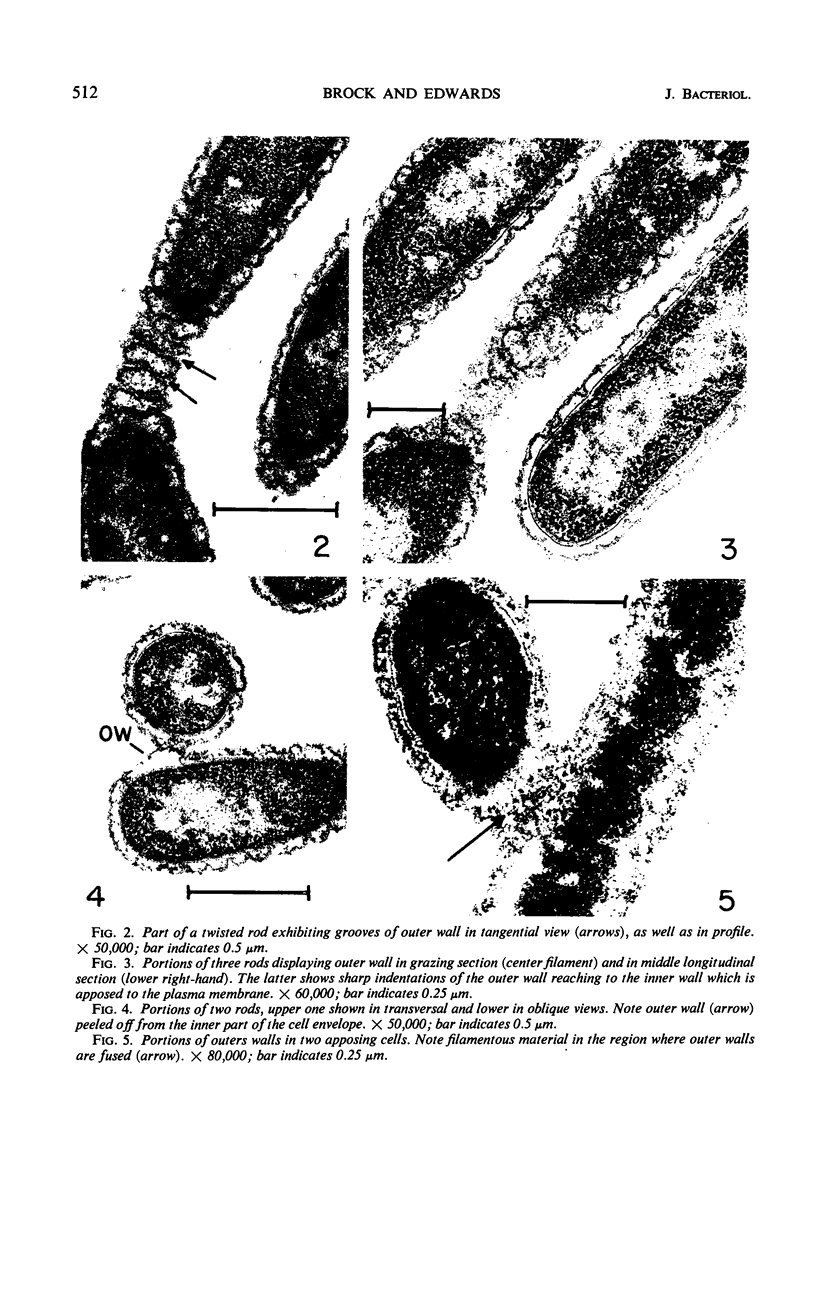
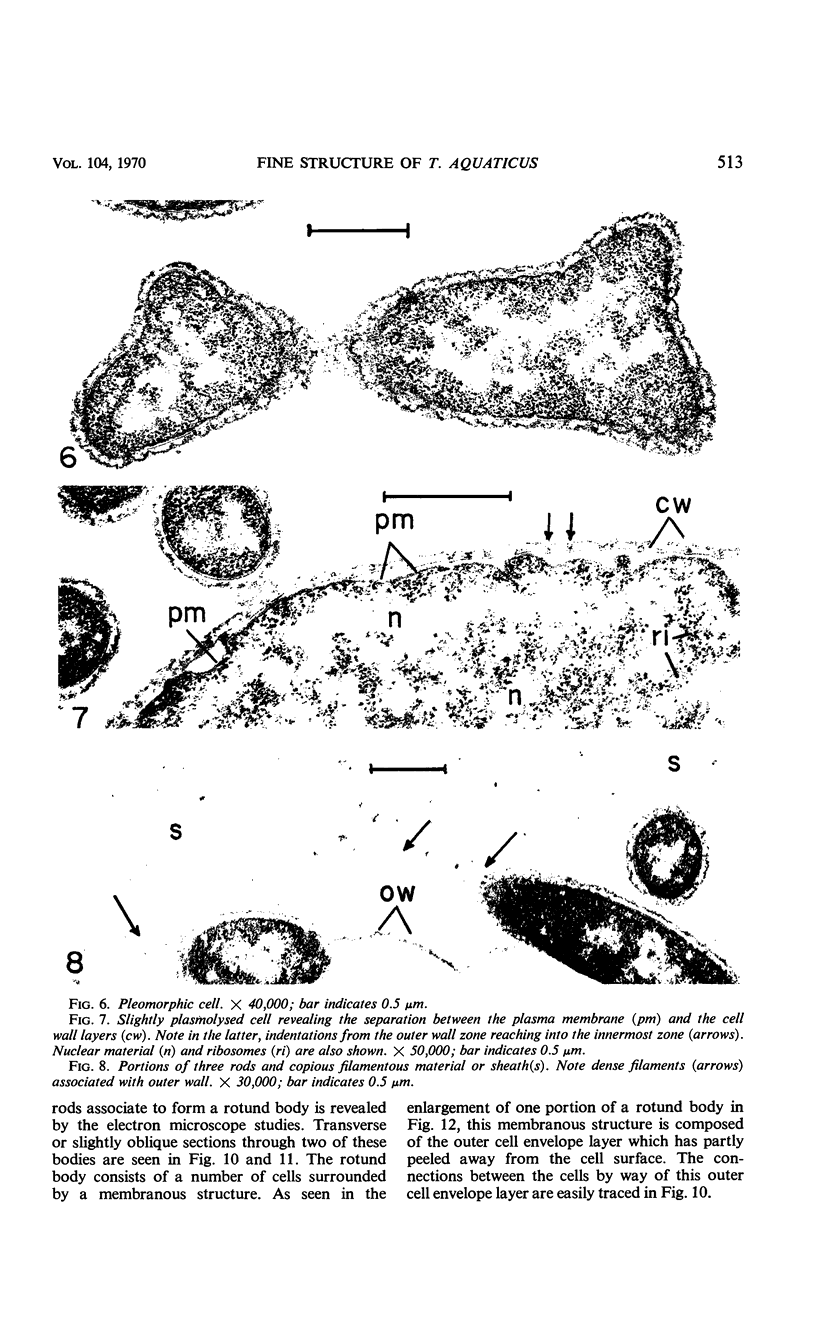
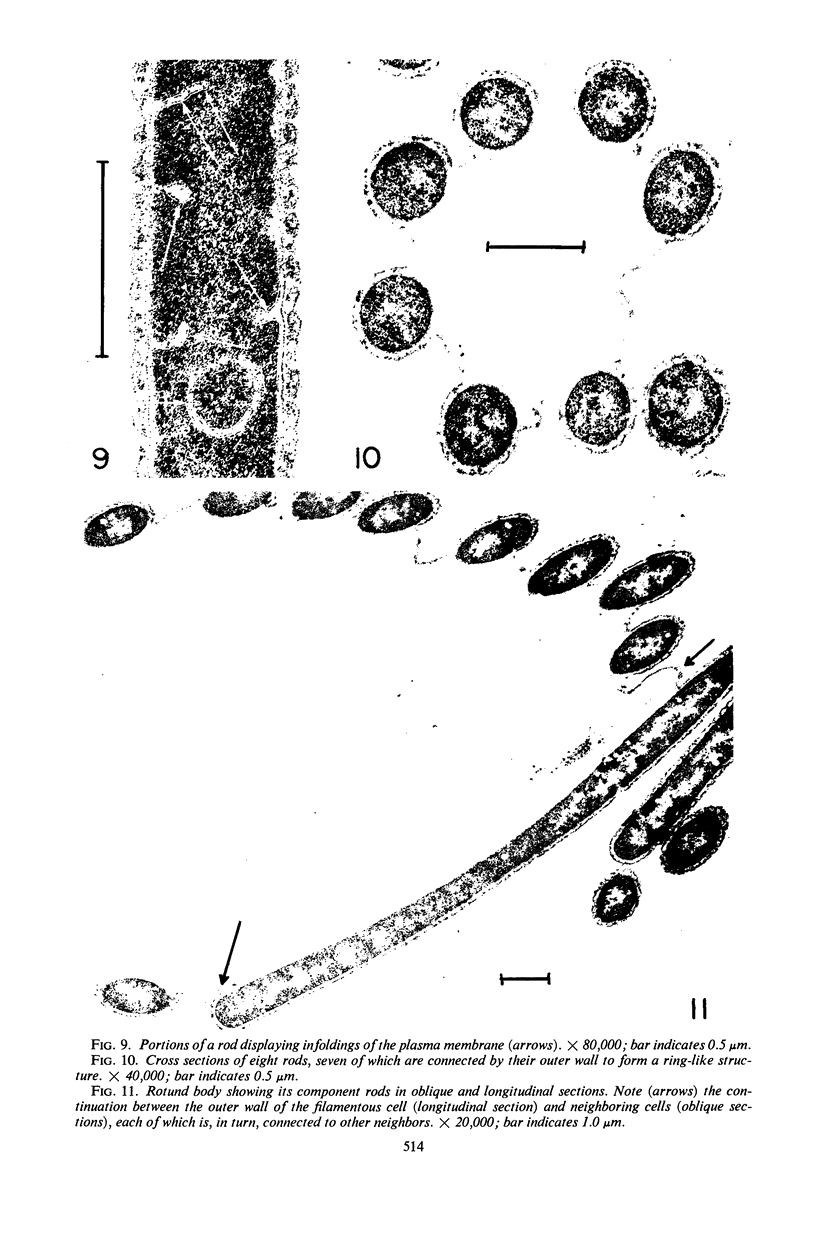
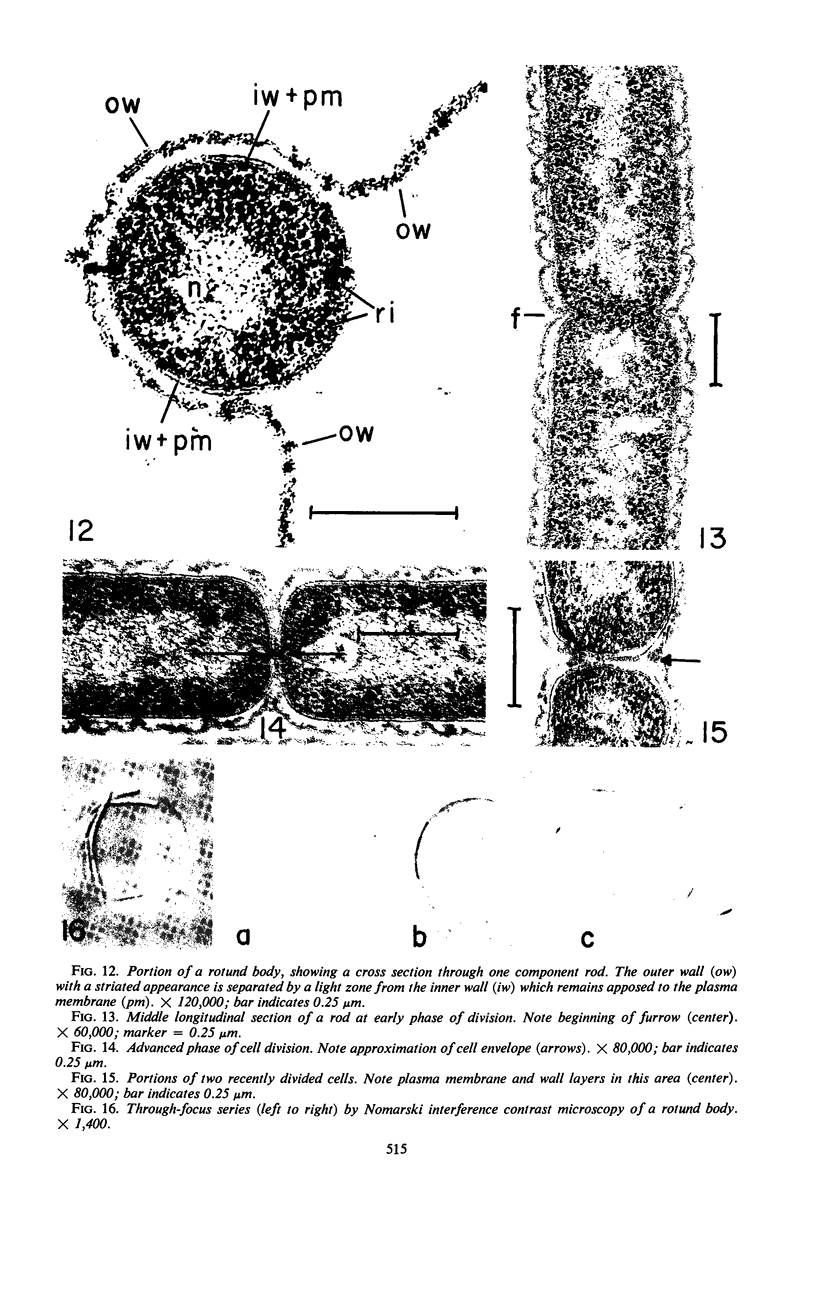
Electron microscopic studies using thin sections revealed that Thermus aquaticus has a structure similar to that of most other gram-negative bacteria. The cell envelope is tripartite: plasma membrane, thin middle layer, and a thicker and irregular outer layer. The outer layer appears to be joined to the plasma membrane by a series of connections and, when seen in tangential section, the outer layer appears as a series of parallel bands. The cell division mechanism resembles that of typical gram-negative bacteria. Large spherical bodies designated “rotund bodies” are formed as a result of the association of a number of separate cells. In this association the outer envelope layers of the cells fuse and pull away from the middle layer. The rotund body thus appears as a series of rods, usually lying in parallel around the periphery of the sphere, completely connected by means of the fused outer layer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauman A. J., Simmonds P. G. Fatty acids and polar lipids of extremely thermophilic filamentous bacterial masses from two Yellowstone hot springs. J Bacteriol. 1969 May;98(2):528–531. doi: 10.1128/jb.98.2.528-531.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Response of Cell Walls of Escherichia coli to a Sudden Reduction of the Environmental Osmotic Pressure. J Bacteriol. 1967 Mar;93(3):1104–1112. doi: 10.1128/jb.93.3.1104-1112.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- EDWARDS M. R., STEVENS R. W. FINE STRUCTURE OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 Sep;86:414–428. doi: 10.1128/jb.86.3.414-428.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felter R. A., Colwell R. R., Chapman G. B. Morphology and round body fermation in Vibrio marinus. J Bacteriol. 1969 Jul;99(1):326–335. doi: 10.1128/jb.99.1.326-335.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney R. P., Edwards M. R. Fine Structure of Thiobacillus thiooxidans. J Bacteriol. 1966 Aug;92(2):487–495. doi: 10.1128/jb.92.2.487-495.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Zeikus J. G., Taylor M. W., Brock T. D. Thermal stability of ribosomes and RNA from Thermus aquaticus. Biochim Biophys Acta. 1970 Apr 15;204(2):512–520. doi: 10.1016/0005-2787(70)90171-1. [DOI] [PubMed] [Google Scholar]