Abstract
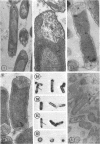
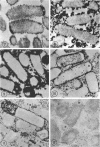
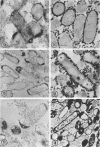
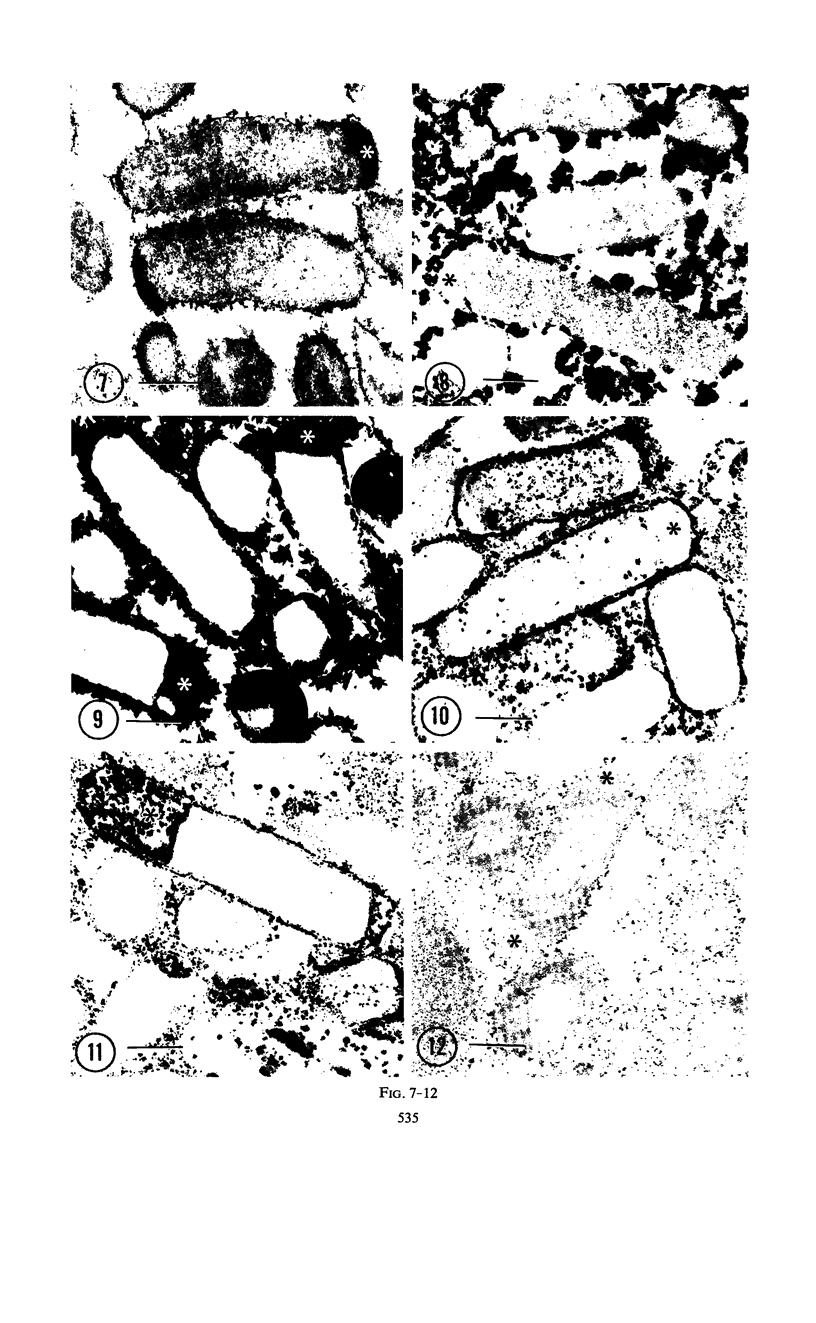
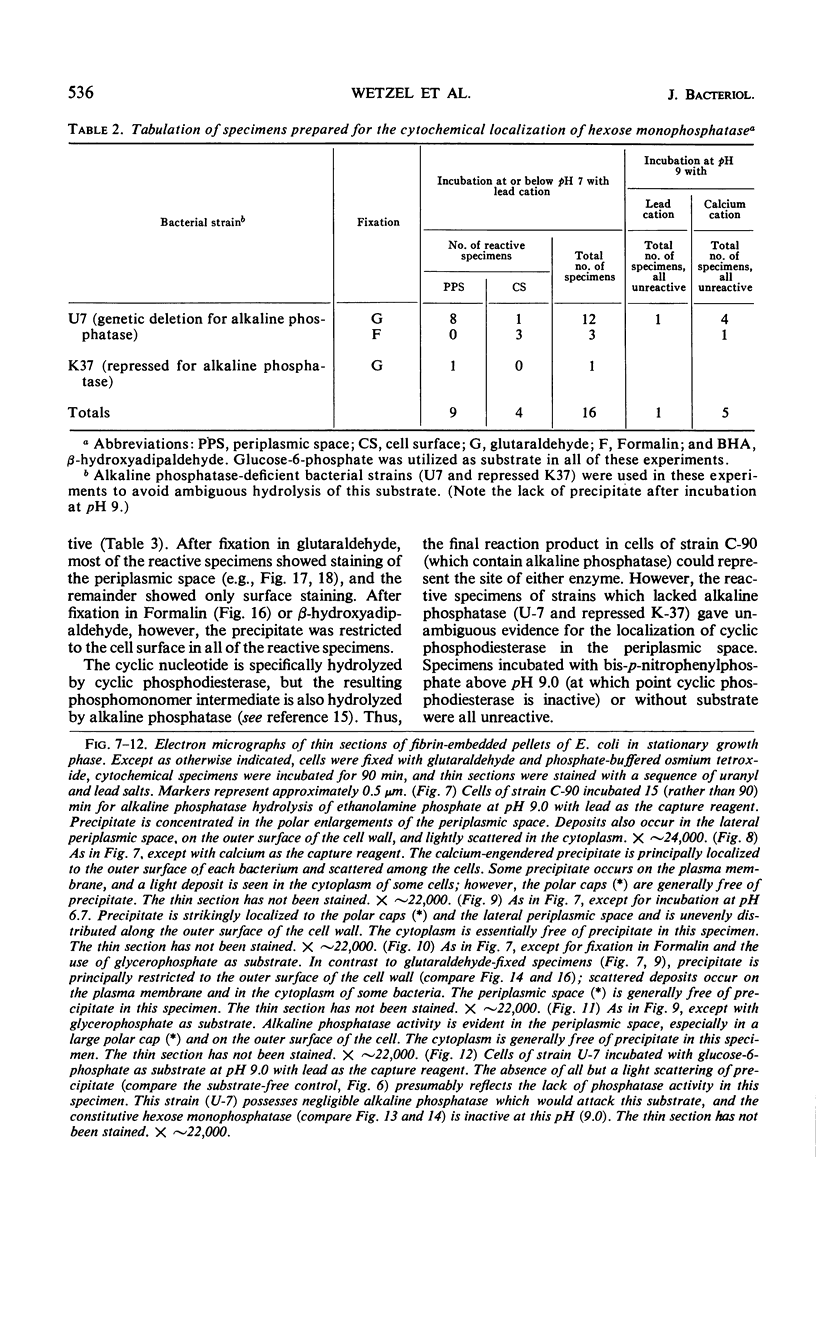
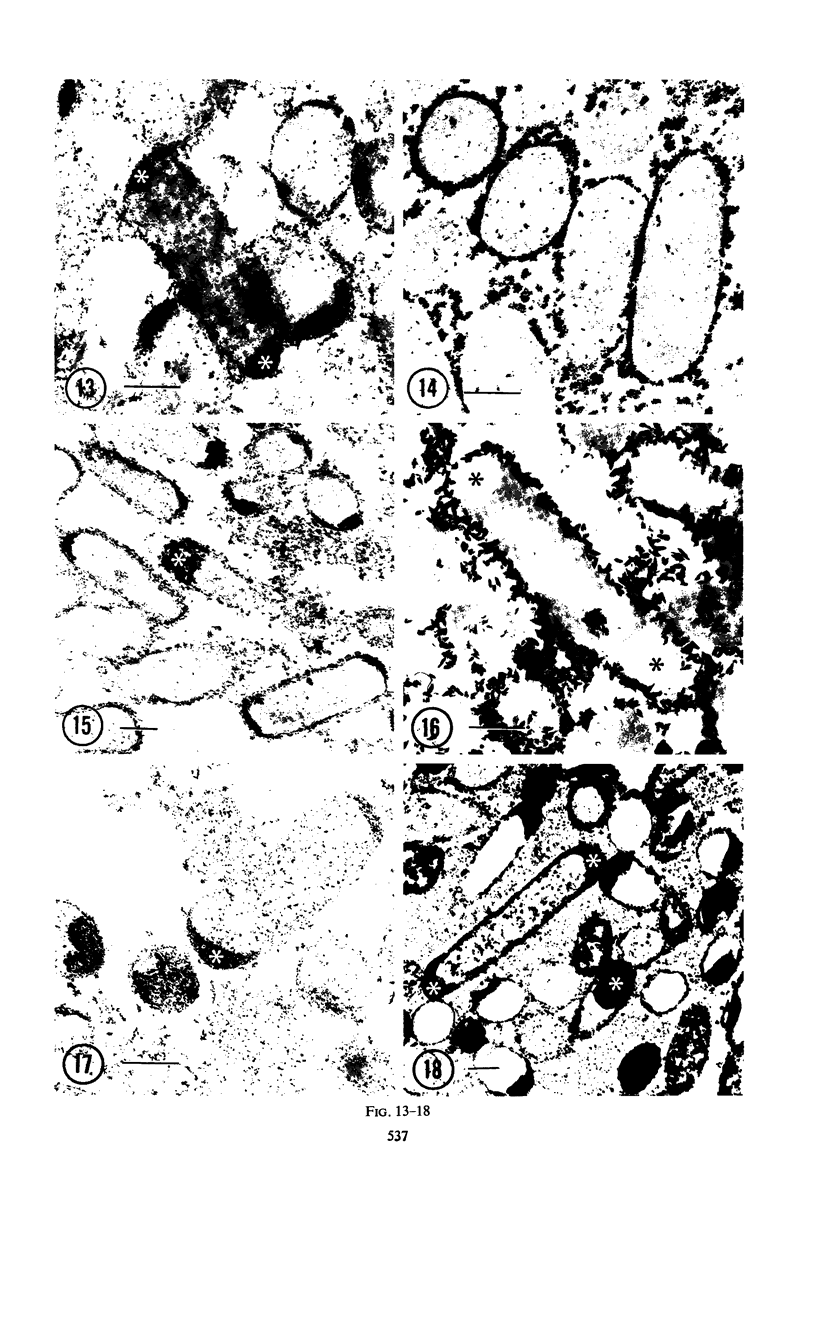
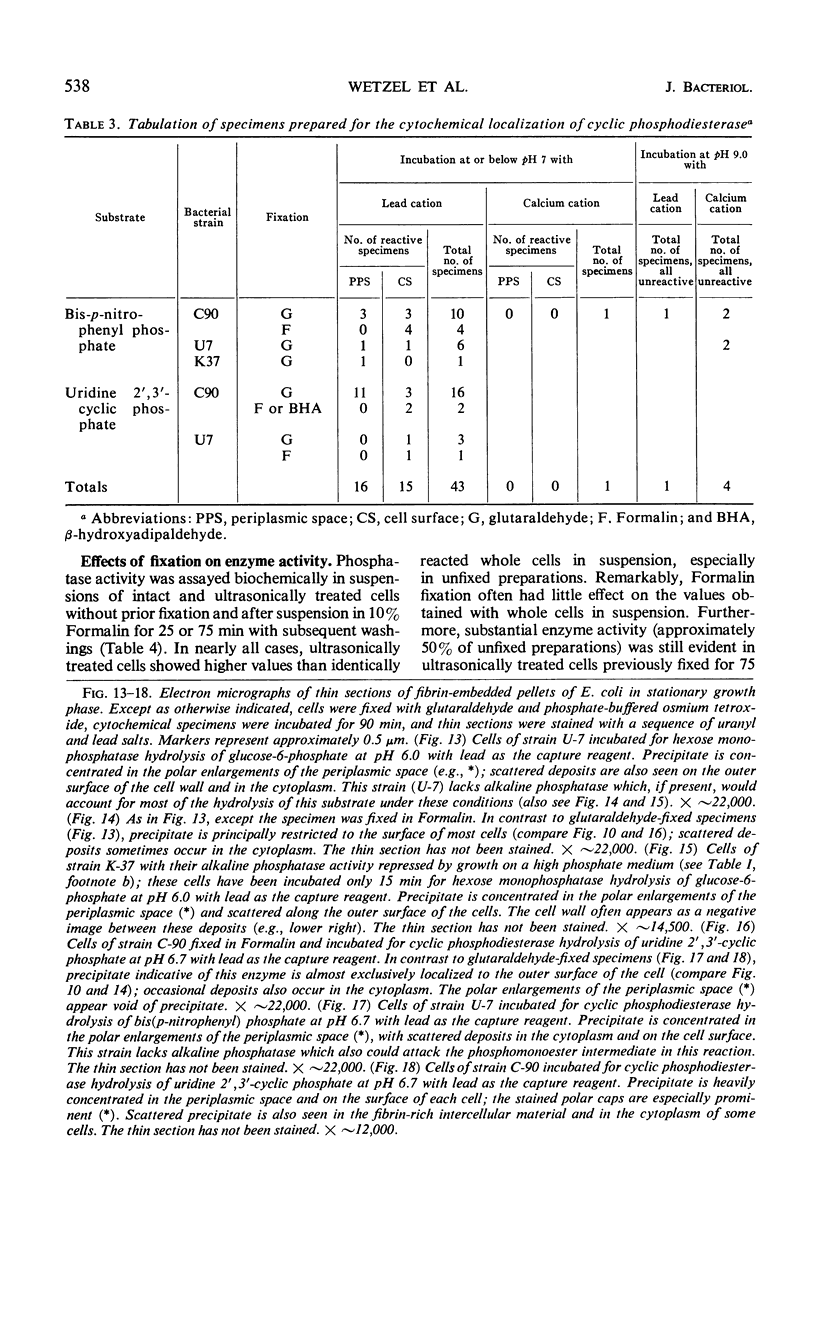
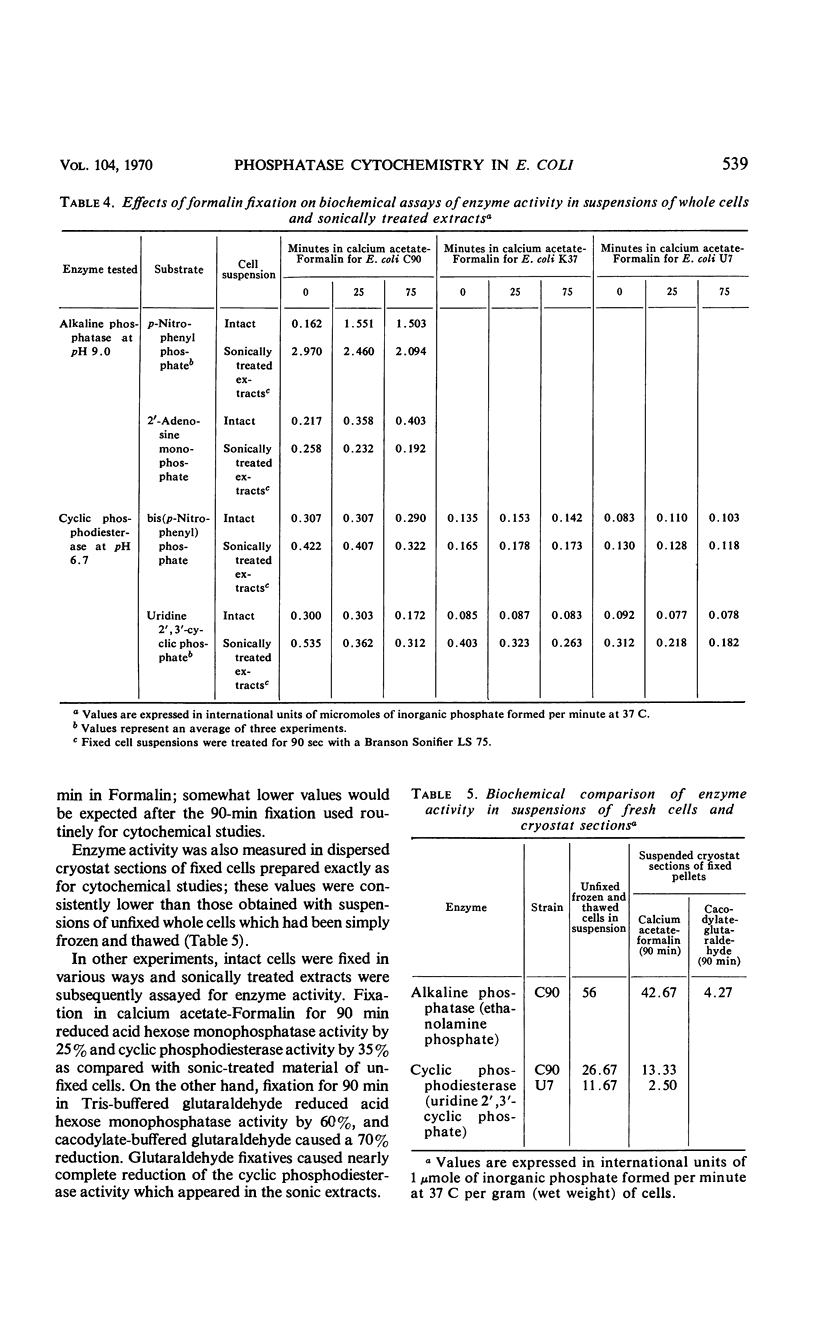
Cytochemical studies of Escherichia coli at the light and electron microscopic levels have revealed alkaline phosphatase, hexose monophosphatase, and cyclic phosphodiesterase reaction products in the periplasmic space and at the cell surface. In preparations for both light and electron microscopy, reaction product filled polar caplike enlargements of the periplasmic space, such as those described in plasmolyzed cells, indicating significant terminal concentrations of these enzymes; dense substance was often seen within these polar caps in morphological specimens. Staining of the bacterial surface was commonly encountered, but could represent artifactual accumulation of precipitate along the cell wall. Alkaline phosphatase was demonstrated with several substrates (ethanolamine phosphate, glycerophosphate, p-nitrophenylphosphate, and glucose-6-phosphate) over a wide pH range in a bacterial strain (C-90) known to be constitutive for this enzyme, whereas strains deficient in this enzyme (U-7, repressed K-37), showed no activity with these substrates. Hexose monophosphatase and cyclic phosphodiesterase activities were characterized by reaction-product deposition with specific substrates at acid or neutral, but not at alkaline, pH in strains of E. coli lacking alkaline phosphatase (U-7 and repressed K-37). Fixation in Formalin or the use of calcium as a capture reagent seemed to interfere with periplasmic staining in cells prepared for electron microscopy. Formalin fixation had little effect on biochemical assays of the phosphatase activity of intact cells in suspension, but partially reduced the activity evident in sonically treated extracts or in suspensions of dispersed cryostat sections. Glutaraldehyde treatment impaired enzyme activity more drastically.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Oct;239:3412–3419. [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):793–800. [PubMed] [Google Scholar]
- BIAVA C. IDENTIFICATION AND STRUCTURAL FORMS OF HUMAN PARTICULATE GLYCOGEN. Lab Invest. 1963 Dec;12:1179–1197. [PubMed] [Google Scholar]
- BLADEN H. A., WATERS J. F. ELECTRON MICROSCOPIC STUDY OF SOME STRAINS OF BACTEROIDES. J Bacteriol. 1963 Dec;86:1339–1344. doi: 10.1128/jb.86.6.1339-1344.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- CEDERGREN B., HOLME T. On the glycogen in Escherichia coli B; electron microscopy of ultrathin sections of cells. J Ultrastruct Res. 1959 Oct;3:70–73. doi: 10.1016/s0022-5320(59)80016-2. [DOI] [PubMed] [Google Scholar]
- COTA-ROBLES E. H. ELECTRON MICROSCOPY OF PLASMOLYSIS IN ESCHERICHIA COLI. J Bacteriol. 1963 Mar;85:499–503. doi: 10.1128/jb.85.3.499-503.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra A., Leblond C. P. Sites of glycogen synthesis in rat liver cells as shown by electron microscope radioautography after administration of glucose-H3. J Cell Biol. 1966 Jul;30(1):151–175. doi: 10.1083/jcb.30.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Brockman R. W., Heppel L. A. Purification and properties of two acid phosphatase fractions isolated from osmotic shock fluid of Escherichia coli. Biochemistry. 1967 Jun;6(6):1743–1751. doi: 10.1021/bi00858a024. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Wetzel B. K., Heppel L. A. Biochemical and cytochemical evidence for the polar concentration of periplasmic enzymes in a "minicell" strain of Escherichia coli. J Bacteriol. 1970 Oct;104(1):543–548. doi: 10.1128/jb.104.1.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966 Dec;30(4):772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hogg R. W., Englesberg E. L-arabinose binding protein from Escherichia coli B-r. J Bacteriol. 1969 Oct;100(1):423–432. doi: 10.1128/jb.100.1.423-432.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Cell wall and cytoplasmic membrane of Escherichia coli. J Biophys Biochem Cytol. 1958 May 25;4(3):323–326. doi: 10.1083/jcb.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. BACTERIAL PROTOPLASTS INDUCED BY PENICILLIN. Proc Natl Acad Sci U S A. 1956 Sep;42(9):574–577. doi: 10.1073/pnas.42.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D. Selection of sucrose-dependent Escherichia coli to obtain envelope mutants and fragile cultures. Science. 1966 Aug 19;153(3738):892–894. doi: 10.1126/science.153.3738.892. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Rosenthal A. S. Pitfalls in the use of lead ion for histochemical localization of nucleoside phosphatases. J Histochem Cytochem. 1968 Aug;16(8):530–539. doi: 10.1177/16.8.530. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Nichoalds G. E., Oxender D. L. Cellular localization of leucine-binding protein from Escherichia coli. Science. 1968 Jul 12;161(3837):182–183. doi: 10.1126/science.161.3837.182. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Robbie J. P., Wilson T. H. Transmembrane effects of beta-galactosides on thiomethyl-beta-galactoside transport in Escherichia coli. Biochim Biophys Acta. 1969 Mar 11;173(2):234–244. doi: 10.1016/0005-2736(69)90107-2. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Secretion of alkaline phosphatase subunits by spheroplasts of Escherichia coli. J Bacteriol. 1968 Sep;96(3):727–733. doi: 10.1128/jb.96.3.727-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOELZ H. SITES OF ADENOSINE TRIPHOSPHATASE ACTIVITY IN BACTERIA. J Bacteriol. 1964 Oct;88:1196–1198. doi: 10.1128/jb.88.4.1196-1198.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelz H., Ortigoza R. O. Cytochemistry of phosphatases in Myxococcus xanthus. J Bacteriol. 1968 Oct;96(4):1357–1365. doi: 10.1128/jb.96.4.1357-1365.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Horn R. G. Fine structural localization of acid and alkaline phosphatases in cells of rabbit blood and bone marrow. J Histochem Cytochem. 1967 Jun;15(6):311–334. doi: 10.1177/15.6.311. [DOI] [PubMed] [Google Scholar]
- Wilson O. H., Holden J. T. Arginine transport and metabolism in osmotically shocked and unshocked cells of Escherichia coli W. J Biol Chem. 1969 May 25;244(10):2737–2742. [PubMed] [Google Scholar]