Abstract
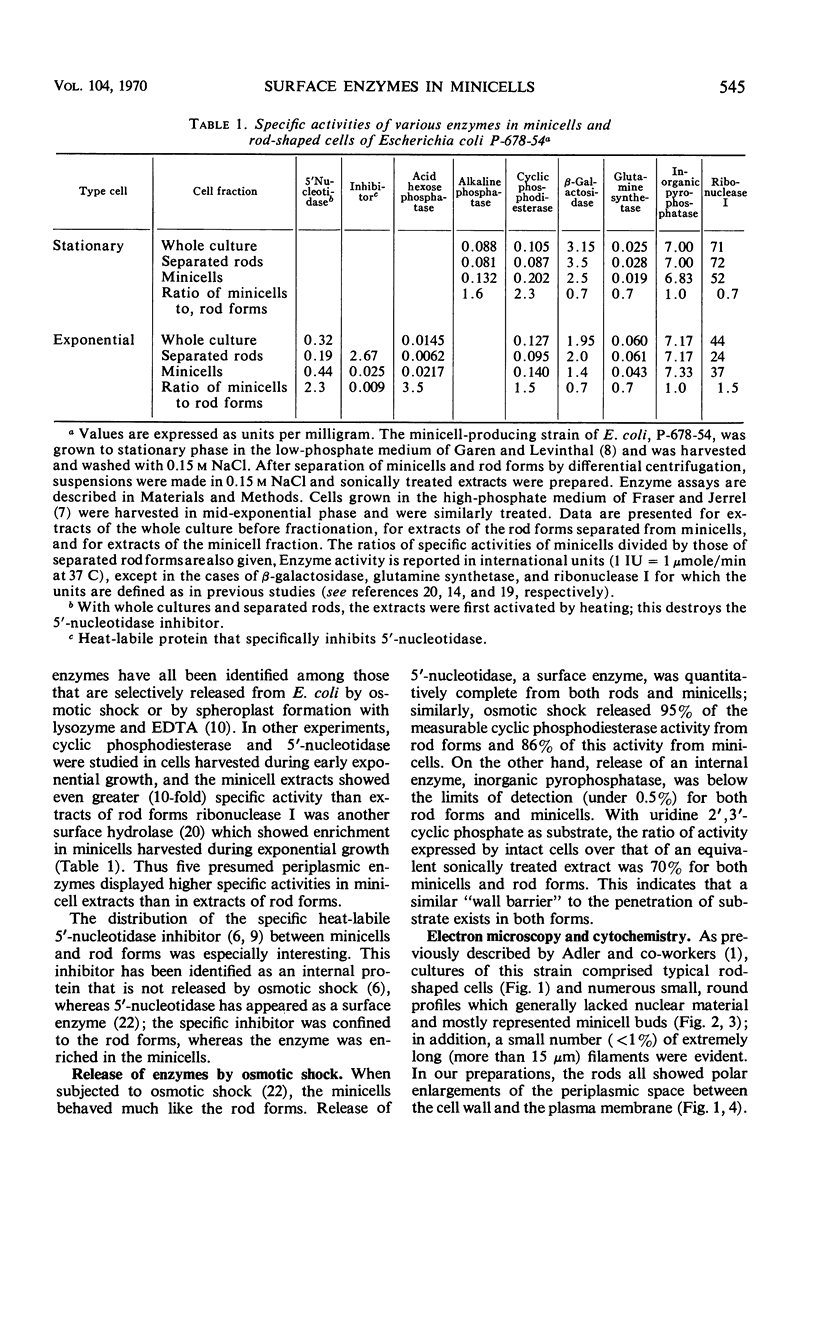
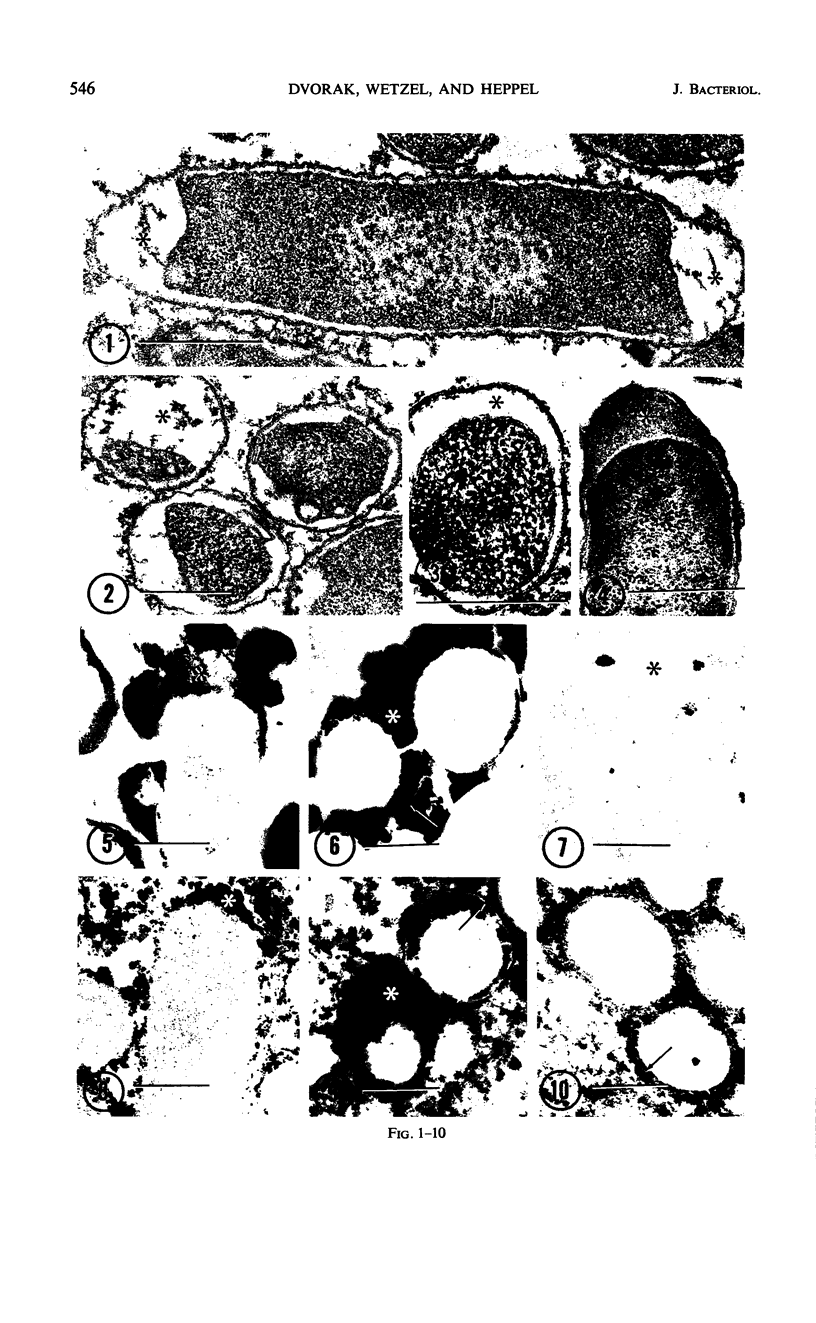
A number of “surface” enzymes of Escherichia coli (i.e., among those selectively released by osmotic shock) all displayed higher specific activities in extracts of minicells than in extracts of typical rod forms; these enzymes included alkaline phosphatase, cyclic phosphodiesterase, acid hexose monophosphatase, 5′-nucleotidase, and ribonuclease I. In addition, alkaline phosphatase, cyclic phosphodiesterase, and acid hexose monophosphatase were cytochemically localized to regions of minicell periplasm that resembled reactive polar enlargements of the periplasm in rod forms. In contrast, a number of “internal” cytoplasmic enzymes (inorganic pyrophosphatase, β-galactosidase, glutamine synthetase, polynucleotide phosphorylase, and ribonuclease II) showed elevated or similar specific activities in extracts of rod forms versus extracts of minicells. A specific heat-labile inhibitor for 5′-nucleotidase, known to occur in the cytoplasm, also showed no enrichment in minicells. These findings indicate that the “surface” enzymes are segregated in vivo into the terminal minicell buds, possibly because these enzymes are concentrated in the polar enlargements of the periplasm in typical rod forms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku Y., Heppel L. A. On the nature of the changes induced in Escherichia coli by osmotic shock. J Biol Chem. 1967 May 25;242(10):2561–2569. [PubMed] [Google Scholar]
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- COTA-ROBLES E. H. ELECTRON MICROSCOPY OF PLASMOLYSIS IN ESCHERICHIA COLI. J Bacteriol. 1963 Mar;85:499–503. doi: 10.1128/jb.85.3.499-503.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. The properties of DNA transferred to minicells during conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:635–641. doi: 10.1101/sqb.1968.033.01.071. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Anraku Y., Heppel L. A. The occurrence of a protein inhibitor for 5'-nucleotidase in extracts of Escherichia coli. Biochem Biophys Res Commun. 1966 Sep 8;24(5):628–632. doi: 10.1016/0006-291x(66)90369-x. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Glaser L., Melo A., Paul R. Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. J Biol Chem. 1967 Apr 25;242(8):1944–1954. [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Josse J. Constitutive inorganic pyrophosphatase of Escherichia coli. 1. Purification and catalytic properties. J Biol Chem. 1966 May 10;241(9):1938–1947. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Cell wall and cytoplasmic membrane of Escherichia coli. J Biophys Biochem Cytol. 1958 May 25;4(3):323–326. doi: 10.1083/jcb.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon H. S., Stadtman E. R. Regulation of glutamine synthetase. X. Effect of growth conditions on the susceptibility of Escherichia coli glutamine synthetase to feedback inhibition. J Bacteriol. 1967 Oct;94(4):949–957. doi: 10.1128/jb.94.4.949-957.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOELZ H. SITES OF ADENOSINE TRIPHOSPHATASE ACTIVITY IN BACTERIA. J Bacteriol. 1964 Oct;88:1196–1198. doi: 10.1128/jb.88.4.1196-1198.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelz H., Ortigoza R. O. Cytochemistry of phosphatases in Myxococcus xanthus. J Bacteriol. 1968 Oct;96(4):1357–1365. doi: 10.1128/jb.96.4.1357-1365.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Dvorak H. F., Heppel L. A. Cytochemical localization of certain phosphatases in Escherichia coli. J Bacteriol. 1970 Oct;104(1):529–542. doi: 10.1128/jb.104.1.529-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]