Abstract
Whether or not the hippocampus participates in semantic memory retrieval has been the focus of much debate in the literature. However, few neuroimaging studies have directly compared hippocampal activation during semantic and episodic retrieval tasks that are well matched in all respects other than the source of the retrieved information. In Experiment 1, we compared hippocampal fMRI activation during a classic semantic memory task, category production, and an episodic version of the same task, category cued recall. Left hippocampal activation was observed in both episodic and semantic conditions, although other regions of the brain clearly distinguished the two tasks. Interestingly, participants reported using retrieval strategies during the semantic retrieval task that relied on autobiographical and spatial information; for example, visualizing themselves in their kitchen while producing items for the category kitchen utensils. In Experiment 2, we considered whether the use of these spatial and autobiographical retrieval strategies could have accounted for the hippocampal activation observed in Experiment 1. Categories were presented that elicited one of three retrieval strategy types, autobiographical and spatial, autobiographical and nonspatial, and neither autobiographical nor spatial. Once again, similar hippocampal activation was observed for all three category types, regardless of the inclusion of spatial or autobiographical content. We conclude that the distinction between semantic and episodic memory is more complex than classic memory models suggest.
Keywords: hippocampus, semantic memory, category production, episodic memory, fMRI, verbal fluency
Introduction
It is clear that the hippocampus plays an essential role in the formation and retrieval of episodic memories. In fact, recent fMRI evidence suggests that the hippocampus participates in the successful retrieval of episodic information and autobiographical events, especially detailed contextual information, even for events that occurred over 20 years ago (Ryan et al., 2001; for review, see Nadel et al., 2007). In contrast, debate continues regarding whether or not the hippocampus is critical for the retrieval of semantic memories, including personal semantics and world knowledge. Much of the evidence on both sides of this debate comes from patients with medial temporal lobe damage. In a recent review, Moscovitch et al. (2006) concluded that retrograde amnesia for semantic memory is either spared completely or confined to a period of about 10 years prior to the head injury, providing that the damage is limited primarily to the hippocampal formation. In contrast, Squire and others (Squire & Zola, 1998; Manns, Hopkins, & Squire, 2003; Luo & Niki, 2002; Squire, Stark, & Clark, 2004) emphasize that at least some amnesics appear to have significant deficits in semantic memory retrieval, even for well-established world knowledge. Semantic memory impairment tends to be more extensive if the damage includes other medial temporal lobe (MTL) and neocortical structures and can reach the same level of deficit as autobiographical memory loss, or even exceed it, in some patients (Bayley et al., 2003, 2005; but see Maguire, 2005).
Neuroimaging evidence is even less consistent than lesion studies regarding hippocampal involvement in episodic, but not semantic, retrieval. A growing number of studies have reported MTL activity during tasks that require access to semantic knowledge, including hippocampal activation during retrieval of public events (Maguire, 2001) and famous faces (Kapur et al., 1995; Leveroni et al., 2000; Bernard et al., 2004), and parahippocampal gyrus activation for famous faces (Haist, Gore, & Mao, 2001) and famous names (Douville et al., 2005). A handful of neuroimaging studies focusing on semantic spatial knowledge have also found activation in hippocampal and adjacent MTL structures. For example, Maguire et al. (1997) reported activation in parahippocampal gyrus when experienced London taxi-drivers were required to find novel routes from one location to another when familiar routes were blocked. Few neuroimaging studies, however, have made a direct comparison between episodic and semantic retrieval tasks that are well matched in other respects, including the type of stimuli presented, the familiarity of the stimuli, and the responses made by the participant, while varying only the source of the retrieved information. In one such study, Maguire et al. (1999) compared yes/no recognition for autobiographical events and public events and found hippocampal activation during both semantic and episodic retrieval, although the level of activation was greater for episodic events. Duzel et al. (1999) also matched conditions carefully in a 2×2 design crossing semantic living/nonliving judgments with recognition old/new judgments. In contrast to Maguire’s (1999) results, however, they found activation in medial temporal lobe only when comparing recognition judgments (old>new) but not semantic judgments.
The discussion of whether or not the hippocampus is involved preferentially in retrieval of semantic and episodic memory assumes that these two types of memory are independent of one another, and this may not be the case. Tulving’s recent work (Tulving, 2005) has outlined the many similarities across these two types of memories. Barsalou and colleagues (Barsalou, 1983, 1988; Barsalou & Sewell, 1985) have suggested that semantic memory is contextually bound to autobiographical information, such that episodic memory is frequently used to generate semantic information. For example, Vallée-Tourangeau et al. (1998) asked participants to generate category exemplars to such common categories as kitchen utensils or food items, and then asked them to describe the strategies they used to generate the items. In over 70% of the common categories, participants reported using a strategy involving a personally familiar context, such as imagining their own kitchen, or walking through the aisles of their neighborhood grocery story (see also Walker & Kintsch, 1985, and Williams & Hollan, 1981). Category production tasks are used widely in neuropsychological evaluations to assess the integrity of semantic memory (Lezak, 2004; Andrewes, 2001). If episodic memory is preferentially used by neurologically normal individuals to generate semantic information in such classic semantic tasks as category production, then patients with hippocampal damage should show impairment on this task. Contrary to this notion, patients with MTL amnesia are most often reported as normal on category production tasks (Schmolck, Kensinger, Corkin, & Squire, 2002). However, one study (Gleissner & Elger, 2001) has reported that patients with lesions restricted to the hippocampal complex generate fewer exemplars during category production than normal individuals or patients with non-hippocampal temporal lobe lesions. In addition, Pihlajamaki et al. (2000) reported fMRI activation in the left hippocampus and adjacent parahippocampal gyrus when normal young subjects generated typical category items.
Clearly, sufficient ambiguity exists in the literature to warrant further investigation of whether or not the hippocampus is engaged during semantic retrieval, particularly given the critical importance of this issue for theories of memory consolidation (for review and discussion, see Moscovitch et al., 2006). In the present study, we used a typical category production task where participants generate exemplars to category names such as modes of transportation or kitchen utensils. In Experiment 1, we compared episodic and semantic versions of the task that differed only in the source of the exemplars that were generated – semantic memory (categorical world knowledge) or episodic memory (a single learning episode that occurred 24 hrs earlier). Note that we define episodic memory here narrowly, referring only to the ability to recall information from a prior event that is bounded by a single spatial-temporal context, and not in the broader sense of requiring an assessment of autonoetic consciousness, or the sense of recollecting, as Tulving (2005) has recently described.
In a second experiment, we compared retrieval within semantic memory for categories that were designed to elicit different types of retrieval strategies. Previous research (Barsalou, 1988; Vallee-Tourangeau et al., 1998) has shown that some, but not all, categories elicit the use of personally familiar spatial contexts (such as their garage or a kitchen) when participants are asked to generate category items. Other categories (such as things that are red or expensive things) do not elicit these contextual strategies. We examined whether or not the presence of spatial and autobiographical strategies during category production would determine whether or not activation is observed in the hippocampus and other MTL regions.
Experiment 1
Methods
Participants
Participants were healthy University of Arizona undergraduate and graduate students who gave written informed consent and received course credit as compensation. All experimental procedures were approved by the University of Arizona institutional review board. Exp. 1 included 10 participants (5 males, 5 females; mean age 24.5, range 19 to 36 yrs; mean years of education 14.2, sd 3). All participants were screened for prior significant head injury, drug or alcohol abuse, psychiatric disorder, and contraindications to MRI.
Materials
Thirty categories were selected from the Battig and Montague (1969) norms and a more recent category normative dataset (Van Overschelde et al., 2004). Common categories with at least 20 exemplars, such as animals, furniture, and kitchen utensils were chosen. For the episodic memory task, seven relatively typical exemplars were chosen from each category excluding the one or two most common responses. The 30 categories were randomly split into two lists, with each list presented equally often in the episodic and semantic retrieval conditions.
Procedure
The experiment included a study phase and a retrieval phase. The study phase occurred 24 hours prior to the fMRI scanning session. Participants came to the laboratory and were required to learn 7 exemplars from each of 15 categories to a criterion of 10 perfect repetitions. We set a strict learning criterion in order to ensure that participants could recall the items without difficulty 24 hrs later. The study phase proceeded as follows. The experimenter read the first category name aloud followed by the 7 exemplars at 2 sec intervals. Participants were instructed to repeat the exemplars aloud, in any order. The experimenter read each subsequent category and exemplar list, and the participant repeated them in any order. No feedback was given after each list. After one complete presentation of the 15 categories, the learning trials were repeated, with two exceptions. First, on each repetition, the categories were presented in a new random order, although the exemplars were always presented in the same order for all learning trials. Second, when the category was named by the experimenter, participants were instructed to recall the list of 7 exemplars in any order. If recall was not perfect, the list of exemplars was read again by the experimenter, and the participant repeated the exemplars aloud. The learning process continued until participants correctly recalled all the exemplars from the 15 categories perfectly, 10 consecutive times. The study session took approximately 1 hour to complete.
Twenty-four hours later, participants underwent MRI scanning. While in the scanner, participants were shown 30 category names, presented in random order using high resolution goggles (Resonance Technologies, Inc.). Each category was preceded by a 1.5 second cue “OLD” or “NEW”, which was then replaced with a category name that remained on the screen for 12 sec. OLD categories were category names for which participants had learned a list of exemplars 24 hrs earlier. For OLD categories, participants were instructed to recall the seven exemplars they had learned the previous day (referred to as the Recall condition). NEW categories were category names that participants had not been exposed to during the study session the previous day. For NEW categories, participants were instructed to generate the first seven items belonging to the category that came to mind (referred to as the Generate condition). In each case, they pressed a mouse button whenever they recalled/generated a word in the category. Because we did not obtain verbal responses in the scanner, the button presses provided some indication that responses for OLD and NEW categories were similar in terms of number of responses and response times. Participants were given 12 seconds to recall/generate items from each category. The 12 sec response window was chosen because pilot data indicated this provided sufficient time for participants to recall the seven items from the memorized list, and also to generate a similar number of exemplars for non-studied categories. A visual-motor control condition was also included 15 times, randomly interspersed between categories. For the Control condition, the letter “X” was presented 7 times within the 12 sec window. The timing of the X’s was randomly jittered across the 12 secs, so that each X was presented for 800–1200 msecs, with interstimulus intervals of 400–600 msecs. This pattern better approximated the button presses made by participants during category production and cued recall conditions. Participants pressed the mouse button each time another X appeared on the screen. Finally, 15 rest periods were interspersed randomly between category and control trials, each lasting 16 seconds during which the word “REST” appeared on the computer screen.
Immediately following the fMRI session, participants were once again asked to recall the lists of category exemplars to ensure that they had recalled the correct items in the scanner. For each category, they were also asked to describe any specific thoughts or strategies that they were aware of as they generated and recalled items.
Image acquisition
Images were acquired on a 1.5T GE full-body echo speed magnet. A set of 3-plane localizer images were first collected in order to align the functional images. Functional data were acquired using a single-shot spiral pulse sequence (Glover & Lee, 1995; Glover & Law, 2001), matrix 64×64, TR = 2000 ms, TE = 40 ms, flip angle = 90, 19 sections, 6 mm, no skip. Sections were placed obliquely, perpendicular to the long axis of the hippocampus in order to minimize partial voluming of the hippocampus, and covered the anterior two-thirds of the brain including the posterior extent of the hippocampus (see Figure 1 showing the placement of the sections). Because the focus of the study was on MTL functioning, we chose to maximize fully volumed data obtained from this region while keeping a reasonably short TR, but at a cost of covering posterior regions including the precuneus, some occipital cortex, and superior parietal cortex. A whole brain high resolution SPGR 3D volume was also obtained for co-registration of the functional dataset, matrix = 256×256, flip angle = 30, TR = 22 ms, TE = 8 ms, FOV = 25 cm, 1.5 mm sections, no skip.
Fig. 1.
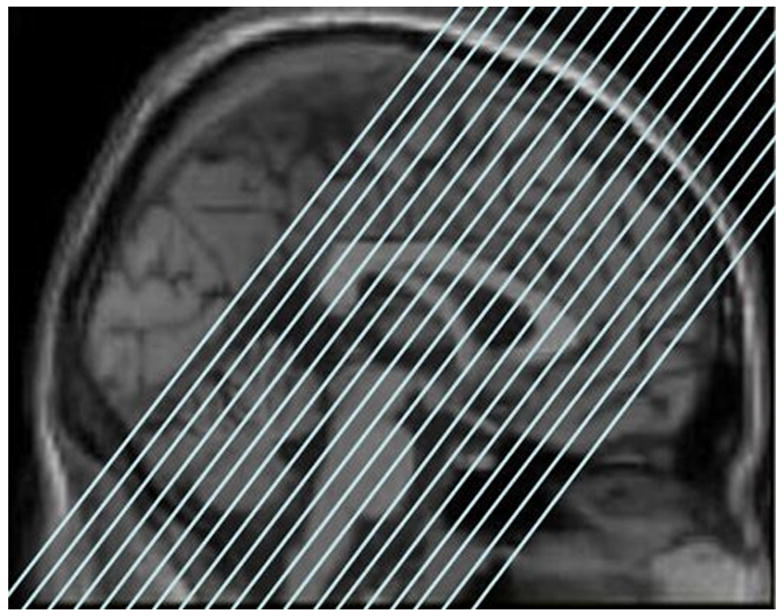
Slice selection for Experiment 1. Note that coverage did not extend to posterior parietal and occipital regions.
Image analysis
Data were analyzed as a blocked design using SPM99 (Wellcome Dept. of Cognitive Neurology; http://www.fil.ion.ucl.ac.uk/spm). ROI analyses were completed using MarsBaR (Brett, Anton, Valabregue, & Poline, 2002) and mean effect sizes were output for further analysis to SPSS, version 14. Images were reconstructed offline and then realigned to the third volume for motion correction. Spatial normalization parameters were estimated by warping each participant’s mean functional image to the standard MNI (Montreal Neurological Institute) EPI template (Ashburner & Friston, 1999). The normalized images were resliced to 3×3×3 mm voxels and smoothed with an isotropic 8mm FWHM Gaussian kernel. The time series in each voxel was highpass-filtered to 1/128 Hz and scaled to a grand mean of 100, averaged over all voxels and scans within the session.
Statistical analyses were performed in two stages. In the first stage, neural activity was modeled by a delta function at stimulus onset. The ensuing BOLD response was modeled by convolving these delta functions with a canonical hemodynamic response function (HRF; Friston et al., 1995). The resulting time courses were down sampled to form covariates in a General Linear Model. Covariates were modeled for the canonical HRFs of the episodic and semantic retrieval conditions described earlier, the control condition, and a single covariate representing the mean (constant) over scans (only canonical HRF covariates were used to make contrasts and move to second level analyses). Contrasts of parameter estimates comprised the data for the second-stage analyses, which treated participants as a random effect. Using methods described by Forman et al. (1995), the statistical threshold was determined by considering both voxel-wise alpha and the extent of contiguous activation. A cluster of 10 contiguous voxels thresholded at p<.01 was required in order to obtain a cluster-level criterion of p<.0001. Stereotactic coordinates were generated in the standard Montreal Neurological Institute (MNI) brain by SPM, and are reported here in MNI space. Mean effect sizes for regions of interest and anatomical boundaries for MTL regions (hippocampus and parahippocampal gyrus) were extracted using anatomical masks from MarsBaR (Brett, Anton, Valabregue, & Poline, 2002).
Results
Behavioral results
Participants were able to recall all the category items perfectly. The responses time data suggest that the two conditions were well matched in terms of number of responses and timing of responses. Within the 12 sec window, they generated an average of 8.0 (sd .3) items in the Generate condition compared to 7 items in the Recall condition, but this difference was not statistically significant. The reaction times were also similar across the two conditions with responses ranging on average between 1606 (sd 78) and 8900 (sd 108) msecs for the semantic condition, and between 1733 (sd 61) and 8216 (sd 94) msecs for the episodic condition (all pairwise t’s<1).
Imaging results
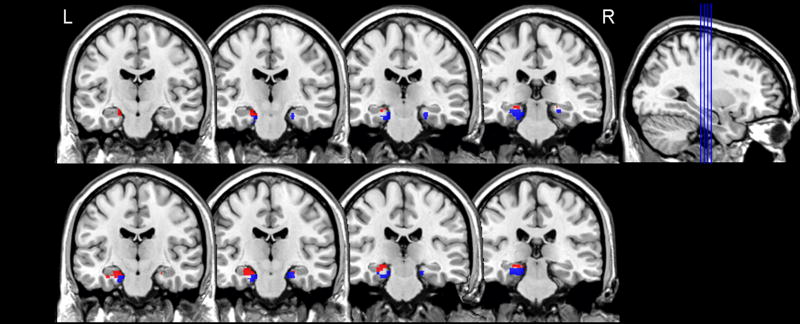
Table 1 shows the MNI coordinates and cluster extents for regions of activation within MTL for the Recall and Generate conditions. Figure 2 shows the strikingly similar placement and extent of activation within MTL for the two conditions. Both conditions elicited activation in left hippocampus and bilateral parahippocampal gyrus.
Table 1.
Regions of activation in the medial temporal lobe for Experiment 1 (cluster p<.0001) and Experiment 2 (cluster p<.005). Laterality (L=left, R=right), MNI coordinates, extent of activation (in voxels; k), and peak voxel t-statistics are provided for each region of interest for the two retrieval tasks in Experiment 1 and for each retrieval strategy in Experiment 2.
| Experiment 1 | MNI | |||||
|---|---|---|---|---|---|---|
| Region
Task |
L/R | x | y | z | k | T |
| Hippocampus | ||||||
| Generate | L | −23 | −19 | −16 | 163 | 7.43 |
| Recall | L | −21 | −19 | −15 | 53 | 4.11 |
| Parahippocampal gyrus | ||||||
| Generate | L | −19 | −26 | −17 | 210 | 8.07 |
| R | 21 | −18 | −19 | 51 | 6.29 | |
| Recall | L | −20 | −25 | −19 | 146 | 5.95 |
| R | 24 | −24 | −18 | 40 | 3.82 | |
| Experiment 2 | ||||||
| Hippocampus | ||||||
| Autobiographical Nonspatial | L | −23 | −29 | −8 | 32 | 2.86 |
| R | - | - | - | - | - | |
| Autobiographical Spatial | L | −23 | −30 | −7 | 101 | 3.90 |
| R | 24 | −26 | −10 | 45 | 3.05 | |
| Nonautobiographical Nonspatial | L | −25 | −30 | −7 | 73 | 3.29 |
| R | 25 | −26 | −10 | 37 | 2.99 | |
| Parahippocampal Gyrus | ||||||
| Autobiographical Nonspatial | L | - | - | - | - | - |
| R | 19 | −29 | −13 | 21 | 3.07 | |
| Autobiographical Spatial | L | −24 | −35 | −12 | 99 | 4.04 |
| R | 21 | −32 | −13 | 84 | 4.28 | |
| Nonautobiographical Nonspatial | L | −18 | −36 | −9 | 18 | 3.14 |
| R | 20 | −31 | −13 | 78 | 3.66 | |
Fig. 2.
Left (L) and right (R) medial temporal lobe activation for the Recall and Generate conditions contrasted with the control task in Experiment 1, cluster p<.0001. Results from activation crossed with hippocampus (red) and parahippocampal gyrus (blue) anatomical regions of interest are presented for each condition separately. Note that activation in the right hippocampus did not survive our extent threshold of 10 contiguous voxels.
In order to further contrast the activation between conditions, we compared mean effect sizes for Recall and Generate conditions in MTL regions that overlapped across conditions. We crossed the combined activation maps for the two conditions with anatomical region of interest maps from MarsBaR (Brett et al., 2002), obtaining mean effect sizes for each participant for the following regions: left hippocampus and left and right parahippocampal cortex. The means for each region were compared with paired t-tests and showed similar mean effect sizes across conditions (all p’s > .05, see Figure 3).
Fig. 3.
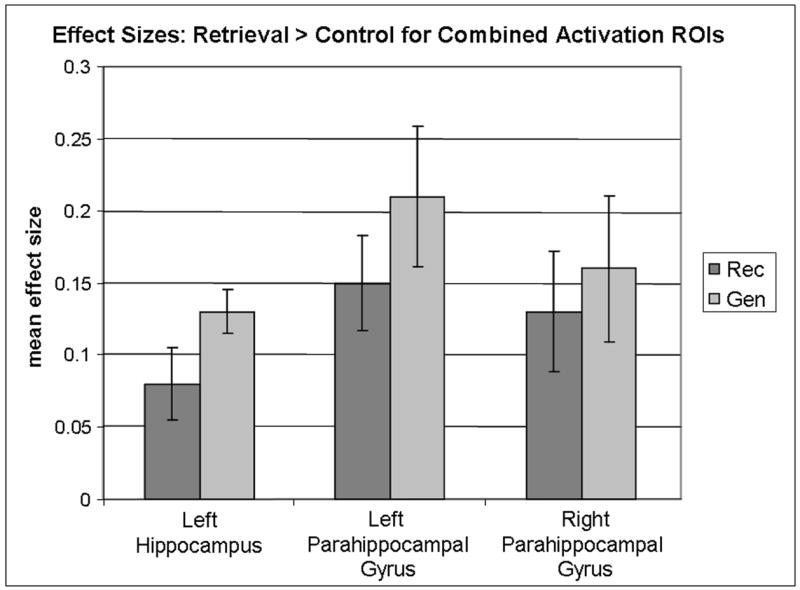
Mean effect sizes for the Recall (Rec) and Generate (Gen) conditions in Experiment 1 contrasted with control within medial temporal lobe (MTL) combined activation regions of interest (ROI), cluster p<.0001. ROIs included all active voxels within left hippocampus and left and right parahippocampal gyrus for both the Rec and Gen conditions. There were no significant differences in mean magnitude of activation between conditions in these regions.
Other brain regions showed some similarity of activation across the two retrieval conditions, but were differentiated by several notable exceptions (see Table 2). Recall elicited activation in left posterior caudate nucleus and the right reticular formation of the midbrain, while Generate showed activation in bilateral inferior frontal gyrus, a more posterior and lateral region of dorsolateral prefrontal cortex, more extensive activation in bilateral basal ganglia, and bilateral thalamus.
Table 2.
Brain regions outside the medial temporal lobe activated by Recall and Generate conditions in Experiment 1 (cluster p<.0001). Laterality (L=left, R=right, B=bilateral), Brodmann Area (BA), MNI coordinates, extent of activation (in voxels; k), and peak voxel t-statistics are given for regions of overlap as well as regions specific to each condition. Medial prefrontal cortex includes the anterior cingulate extending to medial frontal gyrus; ventrolateral prefrontal cortex includes inferior frontal gyrus; dorsolateral prefrontal cortex includes the superior frontal gyrus extending to middle frontal gyrus; basal ganglia includes regions of both the putamen and globus pallidus; fusiform gyrus extents into lingual gyrus and occipital cortex; midbrain refers to the dorsal brainstem extending from the reticular formation to the cerebral and cerebellar peduncles; and posterior caudate extends into superior thalamus.
| Condition | MNI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region Task |
L/R | BA | x | y | z | k | T | ||
| Recall and Generate | |||||||||
| Medial Prefrontal Cortex | B | 32 | −4 | 34 | 22 | ||||
| Recall | 1665 | 7.40 | |||||||
| Generate | 2134 | 8.82 | |||||||
| Ventrolateral Prefrontal Cortex | L | 47 | −29 | 30 | −10 | ||||
| Recall | 62 | 7.92 | |||||||
| Generate | 27 | 3.91 | |||||||
| Dorsolateral Prefrontal Cortex | L | 6/8 | −26 | 16 | 56 | ||||
| Recall | 340 | 6.11 | |||||||
| Generate | 56 | 3.79 | |||||||
| Basal Ganglia | L | - | −16 | −1 | 9 | ||||
| Recall | 95 | 3.68 | |||||||
| Generate | 415 | 4.15 | |||||||
| Midbrain | L | - | −6 | −22 | −20 | ||||
| Recall | 208 | 9.17 | |||||||
| Generate | 117 | 5.39 | |||||||
| Posterior Cingulate | L | 29 | −4 | −50 | 3 | ||||
| Recall | 44 | 6.38 | |||||||
| Generate | 230 | 8.86 | |||||||
| R | 29 | 7 | −47 | 3 | |||||
| Recall | 38 | 7.61 | |||||||
| Generate | 62 | 5.05 | |||||||
| Fusiform Gyrus | L | 19 | −30 | −73 | −21 | ||||
| Recall | 34 | 4.36 | |||||||
| Generate | 80 | 5.12 | |||||||
| R | 19 | 21 | −80 | −18 | |||||
| Recall | 36 | 4.14 | |||||||
| Generate | 22 | 3.47 | |||||||
| Recall Only | |||||||||
| Posterior Caudate | L | - | −10 | −5 | 18 | 16 | 3.91 | ||
| Midbrain | R | - | 7 | −20 | −23 | 45 | 5.50 | ||
| Generate Only | |||||||||
| Ventrolateral Prefrontal Cortex | R | 47 | 33 | 26 | 1 | 21 | 4.60 | ||
| Dorsolateral Prefrontal Cortex | L | 8 | −45 | 8 | 47 | 63 | 4.98 | ||
| Basal Ganglia | R | - | 14 | 7 | 1 | 78 | 5.37 | ||
| Thalamus | B | - | −5 | −10 | 3 | 90 | 4.29 | ||
Discussion
The results provide evidence that the left hippocampus and bilateral parahippocampal gyrus are activated during the retrieval of both episodic and semantic information. Importantly, these tasks were similar in terms of the cues presented and the responses made by the participants. Category production is considered a classic tool for evaluating semantic memory. When patients are impaired on this task, as is often the case for individuals with early Alzheimer’s disease, the result is taken as an indication of a breakdown in the semantic knowledge network (Welsh, Butters, Hughes, Mohs, & Heyman, 1992; Monsch et al., 1994; Chan, Salmon, Butters, & Johnson, 1995). The simplest interpretation of the results would be that the hippocampus is equally involved in the retrieval of both episodic and semantic information. However, given that this finding is inconsistent with the literature on MTL amnesia, we considered an alternative hypothesis focusing on the retrieval strategies adopted by participants during the Generate condition. Following the fMRI session, we asked participants to describe what they were thinking about as they generated and recalled items from the various categories. Consistent with Vallee-Tourangeau et al. (1998), during the Generate condition, participants often reported that they retrieved autobiographical and spatial contextual information that helped them to generate the category exemplars. For example, for the category kitchen utensils, all of the participants described picturing themselves in their own kitchen, looking across the countertops and even opening drawers, as they named items they “saw” in their kitchen. For the category family members, the majority of participants visualized the faces of their family members as they generated exemplars. In contrast, during the Recall condition, participants did not describe such autobiographical and spatial strategies. Instead, they either reported that they were unaware of any particular strategy, or in some cases, they described the semantic organization of the list. For example, one participant said that they recalled the list of animals ordered from smallest to largest. Few participants reported that the episodic lists were organized into a cohesive spatial context.
Although the debriefing information was not collected or coded systematically, these self-reports raised an interesting hypothesis, namely, that the hippocampal activation during the Generate condition may have been driven by the inclusion of autobiographical and spatial contextual information into the retrieval process for category exemplars. To test this hypothesis, we developed categories for an additional study that would reliably elicit different types of strategies. We began with a normative study to identify categories that would or would not elicit autobiographically-relevant information and spatial contextual information. Ideally, this would provide four types of category strategies: autobiographical with spatial context (visualizing my kitchen), autobiographical without spatial context (the faces of my family members), non-autobiographical with spatial context (visualizing a jail cell or the space shuttle), and non-autobiographical without spatial context (either no discernable strategy or relying on semantic strategies alone such as largest to smallest).
We piloted over 60 categories based on work by Barsalou and others (Barsalou, 1983; 1988; Vallee-Tourangeau et al., 1998). Forty undergraduates were asked to produce the first 7 items that came to mind for each category, and then subsequently were asked to describe what they were thinking about as they produced the items. Our goal was to obtain categories for which 80% of participants reported using a similar type of strategy. Three of the four category retrieval strategies were clearly distinguishable from one another using this criterion, including autobiographical with spatial context (AS; things found in a garage, or kitchen utensils), autobiographical without spatial context (AN; things worn on the feet, high school classes), and neither autobiographical nor spatial context (NN; precious stones, things that are red). However, we could not elicit categories where participants used strategies that were clearly not autobiographical but contained a single spatial context. For example, such categories as things found in a jail cell would presumably draw on knowledge of a context that most undergraduates had not yet experienced personally. While participants regularly described visualization of a spatial context, they often described quasi-autobiographical experiences such as imagining a jail cell in a movie that they had watched recently. In order to keep the strategy types clearly separated from one another, we compared only three types, AS, AN, and NN. In order to further ensure that the AS and AN categories drew on autobiographical strategies to differentiate them from the NN condition, the category names included the pronouns “your” and “you” where appropriate, such as things in your garage, or things worn on your feet.
If the use of a spatial context is driving the hippocampal activation observed in Experiment 1, we would expect to see activation only for the AS condition, but not AN or NN categories. Alternatively, any autobiographical content, spatial or otherwise, may result in hippocampal activation. By this view, hippocampal activation should be observed for AS and AN, but not NN categories. Finally, if the hippocampus is important for any semantic retrieval task, then activation in MTL should be evident for all three category conditions, AS, AN, and NN.
Experiment 2
Methods
Participants
Participants included University of Arizona undergraduate and graduate students who gave written informed consent and received course credit as compensation. Exp. 2 included 14 participants (5 males, 9 females; mean age 24.8, range 18 to 40 yrs; mean years of education 15.7, sd 2.9). All participants were screened for prior significant head injury, drug or alcohol abuse, psychiatric disorder, and contraindications to MRI.
Materials
A total of 39 categories obtained from the pilot study described earlier were included in the study. These categories were likely to draw on the three types of strategies described earlier, autobiographical with spatial context (AS), autobiographical without spatial context (AN), and neither autobiographical or spatial (NN). Thirteen categories were included in each condition.
Procedure
Prior to scanning, participants were given several practice categories and were instructed simply to generate as many items belonging to the category as they could think of, and to press the mouse button in their right hand each time they thought of a new category item. While in the scanner, participants were presented visually with a category name and were given 12 secs to generate items from each category. All 39 categories were presented in random order during a single fMRI scan. Thirteen blocks of a visual-motor control condition, described in Experiment 1 (seven X’s jittered within a 12 sec window), were also randomly inserted between categories. Each category and Control block was separated by a 4 second rest period in which the word “REST” appeared.
After the functional scan was completed, participants were debriefed in order to obtain information about the strategies that they used to generate items for each category. Although the categories were developed to elicit particular strategy types, individual differences did occur, and we therefore identified the specific strategy type used for each category based on the participants’ own responses, rather than relying on the normative data. In addition, occasionally a category was excluded when a participant could not describe an unambiguous retrieval strategy. Thus, for each participant, the number of categories included in the AN, AS, and NN conditions varied. However, no participant had less than 8 categories in each condition. The mean numbers of categories across participants included in each condition were as follows: AN mean = 10.5 (sd 2.1), AS mean = 14.3 (sd 2.4), and NN mean = 13 (sd 2.9). When compared using paired t-tests, significantly fewer categories were included in the AN condition compared to either AS or NN conditions (p’s<.05).
Image acquisition and analysis
Images were acquired using the same acquisition parameters as Experiment 1, with the exception that images were collected horizontally aligned to the anterior-posterior commissural plane, covering the whole brain with 27 sections, 3.5 mm no skip. Image preprocessing and data analyses were carried out with the same methods as described in Experiment 1. Whole brain analyses were carried out at a cluster threshold of p<.0001 which was determined following methods developed by Forman et al. (1995) using a voxel-wise p<.01 and an extent of 10 or more contiguous voxels. However, because our hypotheses were based on finding a lack of activation in one or more category conditions and because we included fewer categories in each condition, we employed a more liberal statistical threshold when assessing effect sizes within the medial temporal lobe regions of interest. A threshold of p<.05 with an extent of 12 or more contiguous voxels provided a cluster alpha of p<.005, consistent with previous imaging studies on medial temporal lobe regions that have also used a less stringent statistical criterion (for example, Dobbins et al., 2003; Addis, Wong, & Schacter, 2007)
Results
Medial temporal lobe regions
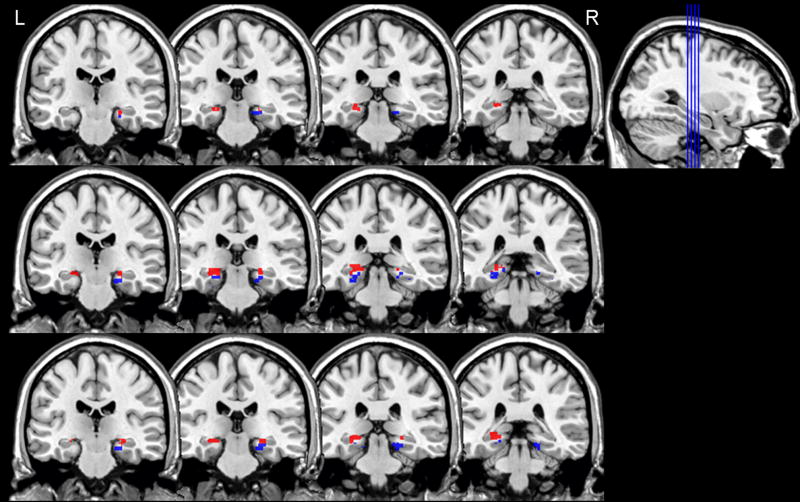
Table 1 shows the MNI coordinates and cluster extents for regions of activation within the MTL for the three strategy conditions. Figure 4 shows the placement and extent of activation for each of the three strategy conditions compared to the Control condition within the MTL. The distribution of activation appears similar for the three conditions and consistent with the pattern of activation observed in Experiment 1 during category production. Activation was observed in both hippocampus and parahippocampal gyrus for all three category conditions, even in the NN category condition where participants reported that they are unaware of any strategy beyond the semantic characteristics of the items.
Fig. 4.
Left (L) and right (R) medial temporal lobe activation for each retrieval strategy contrasted with the control task in Experiment 2, cluster p<.005; Autobiographical Nonspatial (AN), Autobiographical Spatial (AS), and Nonautobiographical, Nonspatial (NN). Results from activation crossed with hippocampus (red) and parahippocampal gyrus (blue) anatomical regions of interest are presented for each condition separately. Note that for the AN condition, activation in the right hippocampus did not survive our extent threshold of 12 contiguous voxels.
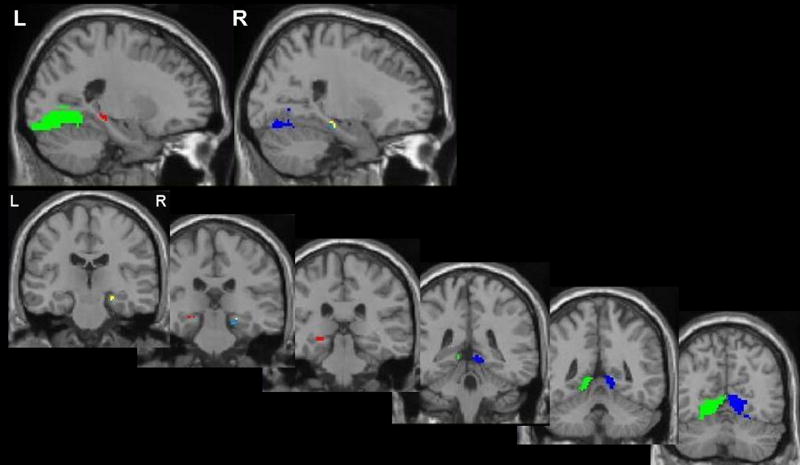
In order to make direct comparisons between the three category conditions within the MTL, activation maps combining activation across all three conditions were crossed with anatomical ROI masks obtained from MarsBaR, providing the mean effect size for each participant from left and right hippocampus, left and right posterior parahippocampal gyrus extending posteriorly to fusiform gyrus and lingual gyrus, and an additional cluster in anterior right parahippocampal gyrus (see Figure 5 for ROIs). Effect sizes were compared across the three strategy conditions in each region using a one-way ANOVA with followed paired t-tests. The results are depicted in Figure 6. First, significant activation was obtained for all three conditions in right and left hippocampus (but activation in right hippocampus for the AN condition did not survive our extent threshold of 12 contiguous voxels), and the mean effect size in these regions did not differ across the category types, F’s<1. This was also true in the anterior right parahippocampal gyrus, F<1. In the more posterior regions incorporating posterior parahippocampal gyrus and fusiform gyrus, significantly greater activation was observed bilaterally for the AS categories compared to either of the two other strategy conditions, AN or NN, as indicated by a main effect of category type, F(2, 81) = 4.99, p<01. Follow-up paired t-tests between conditions are included in Figure 6.
Fig. 5.
Combined activation region of interest (ROI) masks for Experiment 2, created from a combination of activation in all three retrieval strategies contrasted with control at cluster p<.005, which were then crossed with anatomical masks of medial temporal lobe regions. Five separate ROIs were used for analysis: left (L) hippocampus = red, right (R) hippocampus = yellow, left posterior cluster = green, a separate cluster in right parahippocampal gyrus = light blue, right posterior cluster = dark blue. Posterior clusters extend across parahippocampal gyrus, lingual gyrus, and fusiform gyrus.
Fig. 6.
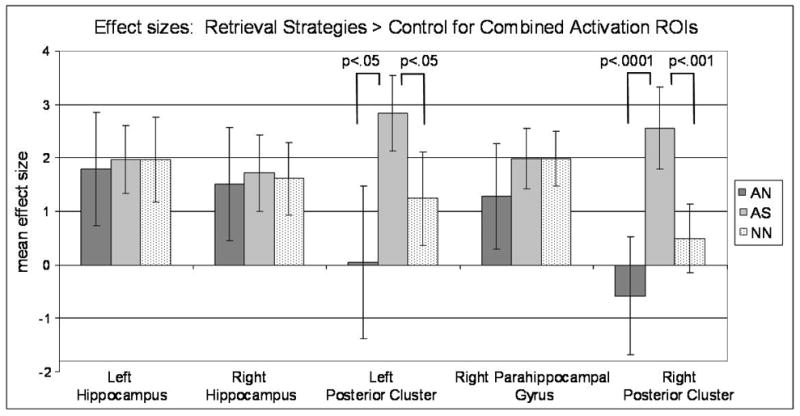
Mean effect sizes for each retrieval strategy contrasted with control in Experiment 2 within medial temporal lobe combined activation regions of interest (ROI), cluster p<.005; Autobiographical Nonspatial (AN), Autobiographical Spatial (AS); Nonautobiographical, Nonspatial (NN). Posterior clusters extend across parahippocampal gyrus, lingual gyrus, and fusiform gyrus. The AS condition had a significantly higher mean magnitude of activation than AN and NN conditions in the left and right posterior clusters.
Other regions of activation
The similarity of activation observed in MTL regions also extended to most, but not all, other brain regions. Table 3A highlights other brain regions showing common clusters of activation across all three strategy conditions, including bilateral dorsomedial prefrontal cortex, left dorsolateral prefrontal cortex, left ventrolateral prefrontal cortex, left thalamus, bilateral retrosplenial/posterior cingulate cortex, and bilateral fusiform/visual cortex extending from the lingual gyrus to fusiform gyrus and cuneus. Voxels of maximal activation within each overlapping cluster were extracted in SPM99.
Table 3.
(A) Brain regions outside the medial temporal lobe activated by all three retrieval strategies and (B) brain regions showing strategy-specific activation in Experiment 2, cluster p<.0001. Laterality (L=left, R=right, B=bilateral), Brodmann Area (BA), MNI coordinates, extent of activation (in voxels; k), and peak voxel t-statistics are given for clusters of activation for each retrieval strategy (AN=Autobiographical Nonspatial, AS=Autobiographical Spatial, NN= Nonautobiographical Nonspatial). Dorsomedial prefrontal cortex includes cingulate gyrus extending to medial prefrontal gyrus; dorsolateral prefrontal cortex includes middle frontal gyrus extending to inferior frontal gyrus; ventrolateral prefrontal cortex includes inferior frontal gyrus extending to insula and superior temporal gyrus; fusiform/visual cortex includes lingual gyrus extending to cuneus, fusiform gyrus, and cerebellum; ventromedial prefrontal cortex includes a region of medial frontal gyrus; lateral parietal cortex includes superior parietal lobule adjacent to the precuneus; superior precuneus regions extend to superior occipital cortex and the cuneus; midbrain refers to activation in the dorsal brainstem extending from the reticular formation to periaqueductal gray; and regions of precentral gyrus extend to middle frontal gyrus.
| A. | |||||||
|---|---|---|---|---|---|---|---|
| Region | MNI | ||||||
| Strategy | L/R | BA | X | y | z | k | T |
| Dorsomedial Prefrontal Cortex | B | 32 | −2 | 24 | 42 | ||
| AN | 294 | 4.89 | |||||
| AS | 966 | 7.45 | |||||
| NN | 633 | 4.01 | |||||
| Dorsolateral Prefrontal Cortex | L | 46 | −50 | 24 | 26 | ||
| AN | 43 | 3.30 | |||||
| AS | 915 | 4.79 | |||||
| NN | 990 | 4.97 | |||||
| Ventrolateral Prefrontal Cortex | L | 47 | −38 | 24 | 2 | ||
| AN | 50 | 3.71 | |||||
| AS | 413 | 5.55 | |||||
| NN | 543 | 6.48 | |||||
| Thalamus | L | - | −2 | −11 | 10 | ||
| AN | 12 | 2.86 | |||||
| AS | 40 | 3.66 | |||||
| NN | 27 | 3.23 | |||||
| Retrosplenial/Posterior Cingulate | L | 29 | −5 | −58 | 8 | ||
| AN | 94 | 4.97 | |||||
| AS | 162 | 7.15 | |||||
| NN | 43 | 4.91 | |||||
| R | 29 | 6 | −48 | 6 | |||
| AN | 19 | 4.38 | |||||
| AS | 69 | 5.19 | |||||
| NN | - | - | |||||
| Fusiform/Visual Cortex | B | 19 | −8 | −66 | −2 | ||
| AN | 699 | 11.20 | |||||
| AS | 749 | 14.96 | |||||
| NN | 807 | 12.27 | |||||
| B. | |||||||
| Strategy | MNI | ||||||
| Region
Strategy |
L/R | BA | x | y | z | k | T |
| AN only | |||||||
| Ventromedial Prefrontal | B | 11 | −4 | 50 | −10 | 70 | 3.93 |
| AS only | |||||||
| Lateral Parietal | L | 7 | −26 | −78 | 44 | 31 | 2.82 |
| Superior Precuneus | L | 7 | −4 | −82 | 46 | 16 | 4.50 |
| NN only | |||||||
| Midbrain | L | - | −4 | −30 | −14 | 125 | 5.25 |
| AS & NN | |||||||
| Precentral Gyrus | L | 6 | −50 | −1 | 50 | ||
| AS | 58 | 3.62 | |||||
| NN | 119 | 3.58 | |||||
In addition to regions of overlap, there were notable regions of activation which distinguished the three strategy conditions. Table 3B highlights regions that differentiated the strategy conditions, showing specific activation for either one or two of the category types, but not all three. These regions were compared directly using mean effect sizes. As discussed previously, the AS condition, in which participants reported visualizing spatial contexts, was associated with greater activation in bilateral posterior parahippocampal gyrus extending into the fusiform gyrus and lingual gyrus. AS also resulted in specific activation in lateral and superior parietal cortex and superior precuneus compared to AN and NN (see Figures 7 and 8). The AN condition elicited activation in this same region of superior precuneus as well, but did not exceed our extent threshold of 10 contiguous voxels. Interestingly, a large region of activation in the ventromedial prefrontal cortex was uniquely observed in the AN condition, with greater activation for AN compared to either AS or NN. The NN condition showed one region within the midbrain, in the dorsal brainstem, that elicited greater activation compared to AS and AN.
Fig. 7.
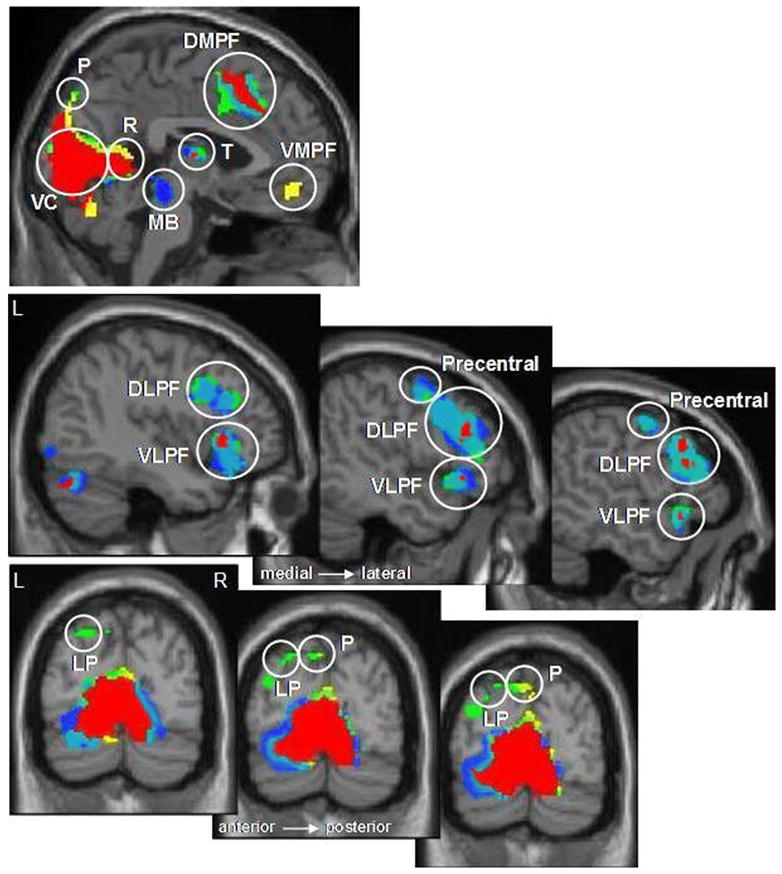
Brain regions outside the medial temporal lobes showing overlapping and strategy-specific activation in Experiment 2, cluster p<.0001. Areas of overlap are shown in red, areas specific to Autobiographical Nonspatial (AN) in yellow, areas specific to Autobiographical Spatial (AS) in green, and areas specific to Nonautobiographical, Nonspatial (NN) in blue. All three retrieval strategies activated the dorsomedial prefrontal cortex (DMPF), left dorsolateral and ventrolateral prefrontal cortex (DLPF, VLPF), thalamus (T), retrosplenial cortex (R), and fusiform/visual cortex (VC). AN retrieval uniquely activated the ventromedial prefrontal cortex (VMPF); AS retrieval uniquely activated left superior lateral parietal cortex (LP) and left superior precuneus (P). The superior precuneus was also activated in the AN condition, but activation did not meet our extent criteria of 10 contiguous voxels (only 8 voxels were observed). The midbrain (MB), a region of dorsal brainstem with activation extending to the inferior colliculus, was activated by NN retrieval only. Left superior precentral gyrus (Precentral) was activated by both AS and NN strategies.
Fig. 8.
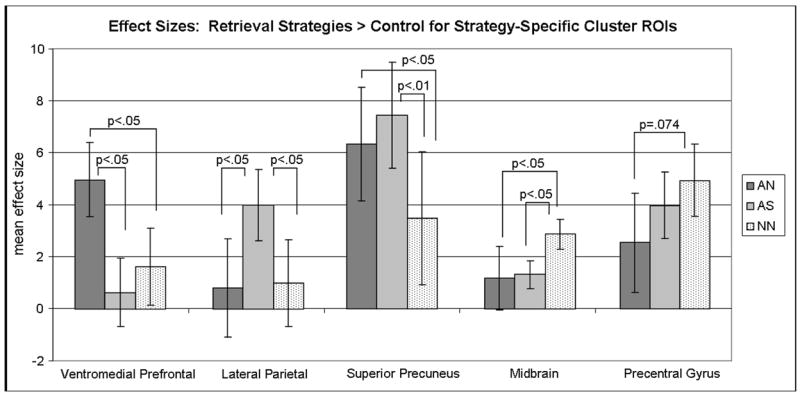
Mean effect sizes for each retrieval strategy in Experiment 2 contrasted with control within strategy-specific cluster regions of interest (ROI), cluster p<.0001; Autobiographical Nonspatial (AN), Autobiographical Spatial (AS); Nonautobiographical, Nonspatial (NN). The AN condition had a significantly higher magnitude of activation than AS or NN in the ventromedial prefrontal cluster; the AS condition had a significantly higher magnitude of activation than AN or NN in the lateral parietal cluster; the AN and AS conditions had significantly higher magnitudes of activation than NN in the superior precuneus cluster (but note that AN activation did not exceed our extent threshold of 10 contiguous voxels); the NN condition had a significantly higher magnitude of activation than AN or AS in the midbrain cluster, a region of dorsal brainstem; and the NN condition had a marginally significant higher magnitude of activation than AN in the precentral gyrus cluster.
Discussion
In summary, the results from Experiment 2 showed a similar pattern of activation for semantic retrieval as observed during category production in Experiment 1. Activation was again evident within the medial temporal lobes as participants generated exemplars for categories. Whether or not the three category types elicited differential activation depended upon the specific region within MTL. Within the hippocampus proper, activation did not differ as a function of whether participants used autobiographical or spatial contextual information as a strategy for retrieval. The posterior parahippocampal gyrus, however, showed significantly greater activation for categories that elicited an autobiographically-relevant spatial context, such as the participant’s kitchen, garage, or local supermarket. AS categories also activated fusiform and lingual gyrus, along with superior parietal cortex. Collectively, these posterior regions have been associated with the processing of complex scenes (Hayes et al., 2004; Burgess, Maguire, Spiers, & O’Keefe., 2001), episodic memory retrieval (Ryan et al., 2001; Shannon & Buckner, 2004; Gilboa et al., 2004), spatial navigation tasks (Maguire, 1997), and retrieval of remote spatial information in amnesics (Rosenbaum et al., 2004), and are consistent with the self-reports of participants of visualizing and sometimes even navigating through personally familiar spaces.
Activation in the superior precuneus was present for both conditions that elicited autobiographical content, AS and AN (but did not meet extent thresholds in the AN condition). This region also has been associated with episodic memory retrieval (Ryan et al., 2001; Shannon & Buckner, 2004; Gilboa et al., 2004), and has been implicated in tasks that relate to judgments regarding the self (Northoff et al., 2006; Cavanna & Trimble, 2006). Ventromedial prefrontal cortex, observed only during retrieval of the AN categories, is also a region that has been associated with self-referential tasks (Northoff et al., 2006; Saxe, Moran, & Scholz, 2006; Johnson et al., 2002), consistent with the notion that participants generated autobiographically-relevant information in order to generate items for categories such as family members.
Of particular interest is the pattern of activation associated with NN categories, where participants are unaware of any specific autobiographical or spatial strategy used to generate the items. Of the three category types, NN categories should represent the “purest” semantic memory task, uncontaminated by episodic or autobiographical content. NN categories were most clearly differentiated from AS and AN in posterior cortical regions including the precuneus, posterior parietal, and fusiform gyrus, and anteriorly in ventromedial prefrontal cortex. Nevertheless, NN categories shared many common regions with both AS and AN categories, including the hippocampus, dorsomedial prefrontal cortex, and retrosplenial cortex, all regions that are also commonly activated during episodic memory retrieval tasks. It appears that even for these categories, exemplar retrieval is mediated, at least in part, by regions in common with episodic memory retrieval.
General Discussion
In studies of verbal fluency, including category production, the literature tends to focus primarily on the critical role of the left inferior frontal cortex and whether or not this frontal region is singular or further separable into phonological and semantic subcomponents (see Costafreda et al., 2006, for review). Discussion of temporal lobe function has emphasized the importance of lateral temporal cortex, rather than medial temporal lobe regions, in category fluency tasks (Baldo et al., 2006, Schmolck et al., 2002). Medial temporal lobe involvement in category production has garnered less attention. However, several studies have found MTL activation during category production. For example, Pihlajamaki and colleagues (2000) found activation in the left medial temporal lobe including the hippocampus and posterior parahippocampal gyrus during generation of exemplars for typical categories such as clothing, food, animals, and plants (see also Hirshorn & Thompson-Schill, 2006, Exp. 2; Mummery, Patterson, Hodges, & Wise, 1996). One reason for fewer reports of MTL activation may be that scans are often limited to the frontal lobe, and thus do not assess all brain regions (Paulesu et al., 1997; Crosson, Sadek, et al., 2001).
Nevertheless, the consistent finding in the literature is that amnesics with MTL damage are generally not impaired on category production, or for that matter, on many other tasks of semantic retrieval. In a recent paper, Schmolck and colleagues (Schmolck et al., 2002) tested 6 amnesic patients with damage either restricted to the MTL or damage that extended past MTL into anterior and lateral temporal lobe. Although some individual patients did poorly on specific semantic retrieval tasks, as a group, deficits were only obvious in the amnesic patients with damage extending into lateral temporal areas.
Given the ability of individuals with hippocampal damage to perform adequately on this task, why do we see hippocampal activation in normal participants performing category production? One possibility is that the hippocampus is utilized when and if it is available and intact, providing a more efficient and flexible way of searching for and retrieving semantic information. By this view, the presentation of the category acts as a cue that activates related information, semantic and episodic alike. Related episodic information, such as familiar places or people, may be used by the participant to generate the semantic material in a sort of bootstrapping of semantic retrieval. The hippocampus may provide support for retrieval and increase efficiency of search, particularly with categories that are less well established, such as categories that are based on organizing principles other than typical semantic relatedness, such as a particular location (things in a garage) or a shared perceptual feature (things that are red). These types of categories have been referred to by Barsalou (1983) as ad hoc categories.
What is special about ad hoc categories? During these tasks, one must use semantic knowledge in a flexible and perhaps relatively novel way, emphasizing characteristics of an item and similarities across items that would otherwise be irrelevant. We hypothesize that, under these conditions, amnesics with medial temporal lobe damage may do less well in generating category members, compared to generation tasks where the organizing principle amongst items is common and overlearned. Amnesics might be relatively intact on classic semantic categories, such as animals or fruits and vegetables, relying primarily on intact non-medial temporal systems, but may have more difficulty when asked to generate exemplars to labels such as things that are expensive or things you take on a camping trip. Our argument here is similar to the notion of degeneracy, discussed by Price and Friston (2002; Friston & Price, 2003), which emphasizes that there are multiple ways in which a complex cognitive task can be accomplished. We are not suggesting that the hippocampus is merely “involved but not necessary” for semantic retrieval. Instead, we propose that multiple retrieval systems are engaged cooperatively, depending upon the requirements of the task, providing complementary routes for retrieval. Understanding the interaction of these systems will probably require the integration of data from studies of both normal and brain-injured individuals.
The concept of semantic and episodic memory as interactive and generative in the normal brain appears to have support not only from cognitive psychology (for example, Barsalou, Huttenlocher, & Lamberts, 1998), but also from evidence demonstrating overlapping brain networks involved in the retrieval of semantic and episodic memory. In a recent paper, Rajah and McIntosh (2005) used structural equation modeling to identify networks that might differentiate semantic and episodic memory retrieval. Instead, separate episodic and semantic models failed to differentiate from one another, with similar patterns of path coefficients for the two retrieval models across tasks. The authors argue that the same memory network was engaged across tasks, and that differences between episodic and semantic retrieval may reflect variation along a continuum of processing during task performance, rather than the output of two independent memory systems. A similar outcome was recently reported by Burianova and Grady (2007), showing overlapping networks of activation during semantic and episodic retrieval, which included regions of left medial temporal lobe.
According to Barsalou, “there are no invariant knowledge structures in memory. Instead, people continually construct unique representations from loosely organized generic and episodic knowledge to meet the constraints of particular contexts” (Barsalou, 1988, p. 236). Instead of focusing on abstracted concepts, Barsalou emphasizes the critical role of instances for generating semantic knowledge. This is an interesting idea, because it presents semantic memory as something that is not simply a stable and accurate record of past learning, but something that is generative, flexible, contextually bound, and subject to revision through novel experience. Semantic memory is generated anew each time it is required, in much the same way as Bartlett (1932) and others (e.g., Nadel, Campbell, & Ryan, 2007) have noted that episodic memories are reconstructed and revised over time and through multiple retrievals. This highlights the similarities between episodic and semantic memories (Tulving, 2005) and stands in contrast to the assumption that semantic memory, at least, is a faithful record of prior knowledge.
Other related memory research has recently demonstrated how semantic and episodic memories can interact with one another. Using a very different paradigm, Westmacott and Moscovitch (2003) showed that episodic memory can contribute to performance on tests of semantic memory. They reported that categorization judgments regarding professions for famous people is faster and more accurate if the name is associated with a recollection that has personal significance to the participant. In the example they cite, Elvis Presley might be associated with a personal visit to Graceland, whereas Frank Sinatra holds no such personal association. Performance therefore favors Elvis Presley, even though both people are equally famous. For another individual, the opposite might well hold true, depending upon their own unique episodic experiences.
These considerations suggest that debates in the field about whether or not episodic and semantic memory are part of the same memory system, subserved by the same neural substrate, miss the point (see Moscovitch et al., 2006, for discussion). Differences observed between episodic and semantic memory tasks may be better understood in terms of the information required for any particular task, given that an individual will use whatever means are available to them, episodic or semantic, in order to solve the problem. Episodic memory tasks, by definition, require the retrieval of contextual information including time and place. Even a recognition task requires that the individual determine not only whether an item has been experienced before (which would include all words in a list) but whether the particular word occurred at a particular time and place. No such requirement is inherent in most semantic memory tasks, where often the task requires a judgment about the semantic qualities of words (such as in a living/nonliving judgment task). Engagement of the hippocampus may depend on the degree to which performance requires (or can benefit from) the retrieval of contextual, spatial, and temporal, information. In the case of normal individuals, category production tasks can be solved by accessing a mix of episodic, autobiographic, and semantic knowledge. For example, if a subject is asked to retrieve items that are found in a restaurant, they may think about restaurants in general, or a restaurant that they go to frequently, but they may be equally likely to visualize the specific restaurant that they were at last night. As we and others have suggested, the hippocampus may best be viewed as a system that automatically uses inputs (in this case, a category cue) to generate linked information, thereby retrieving any appropriate related information.
One important implication of this formulation is that it suggests that the connections between hippocampal (episodic) representations and extrahippocampal stores of semantic information are not lost altogether over time. Rather, some of these connections remain, and can be used in the normal case to retrieve semantic knowledge connected to specific contexts. The fact that there are alternative routes for accessing semantic memory is not surprising. It remains to be seen how flexible these alternative routes are, and under what circumstances they may or may not be sufficient for a task. This formulation is consistent with recent work exploring the fate of contextual coding in animals. A number of studies, mostly using fear conditioning, have shown that shortly after training fear is relatively restricted to the original training context (Wiltgen & Silva, 2007; Winocur, Moscovitch, & Sekeres, 2007). With the passage of time, and no further training, fear will be evinced in a wider range of contexts. This “loss” of context-specificity has typically been viewed as involving the loss of some hippocampal trace that would resist generalizing fear across contexts, but such an interpretation is not mandated by the data. In fact, it is equally possible that a separate, “semantic” trace is created (outside the hippocampus) that connects fear to generic features of the original training context, such as grid floors, or a small box with four walls. This trace would support fear in multiple contexts, but its presence need not involve the loss of the more specific trace supporting fear in the original context. Much as in the human case, rats would have multiple ways to access fear memory, some via the original training episode, others via semantic knowledge about that experience.
The broader implication of this way of thinking concerns the notion of a “memory system”. Perhaps it is no longer sensible to talk about self-contained memory systems, either singular or plural. Rather, we should be talking about the acquisition of various kinds of knowledge (see also Nadel, in press; Lee, Barense, & Graham, 2005), and the subsequent deployment of particular aspects of that knowledge in the service of “memory” tasks. Within this view, all forms of knowledge would be subject to transformation (via consolidation) and updating (via reactivation and reconsolidation), and memory retrieval would involve accessing the appropriate knowledge to fit the task demands. There is neither one nor multiple “memory” systems, only a variety of systems that both process and store different types of information, to be deployed as required.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrewes D. Neuropsychology: From theory to practice. New York: Taylor & Francis; 2001. [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12(6):896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Ad hoc categories. Mem Cognit. 1983;11(3):211–227. doi: 10.3758/bf03196968. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Sewell DR. Contrasting the representation of scripts and categories. Journal of Memory and Language. 1985;24:646–665. [Google Scholar]
- Barsalou LW. The content and organization of autobiographical memories. In: Neisser U, Winograd E, editors. Remembering reconsidered: Ecological and traditional approaches to the study of memory. Cambridge University Press; 1988. pp. 193–243. [Google Scholar]
- Barsalou LW, Huttenlocher J, Lamberts K. Basing categorization on individuals and events. Cognit Psychol. 1998;36(3):203–272. doi: 10.1006/cogp.1998.0687. [DOI] [PubMed] [Google Scholar]
- Bartlett FC. Remembering. Cambridge: Cambridge University Press; 1932. [Google Scholar]
- Battig WF, Montague WE. Category norms for verbal items in 56 categories: a replication and extension of the Connecticut category norms. J Exp Psychol. 1969;80:1–46. [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38(1):135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Squire LR. Failure to acquire new semantic knowledge in patients with large medial temporal lobe lesions. Hippocampus. 2005;15(2):273–280. doi: 10.1002/hipo.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard FA, Bullmore ET, Graham KS, Thompson SA, Hodges JR, Fletcher PC. The hippocampal region is involved in successful recognition of both remote and recent famous faces. Neuroimage. 2004;22(4):1704–1714. doi: 10.1016/j.neuroimage.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox; Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O’Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14(2):439–453. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Burianova H, Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. J Cogn Neurosci. 2007;19(9):1520–1534. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- Chan AS, Salmon DP, Butters N, Johnson SA. Semantic network abnormality predicts rate of cognitive decline in patients with probable Alzheimer’s disease. J Int Neuropsychol Soc. 1995;1(3):297–303. doi: 10.1017/s1355617700000291. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27(10):799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gokcay D, Mohr CM, Auerbach EJ, et al. Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. J Cogn Neurosci. 2001;13(2):272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HG, Wagner AD, Schacter DL. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, et al. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43(5):693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Duzel E, Cabeza R, Picton TW, Yonelinas AP, Scheich H, Heinze HJ, et al. Task-related and item-related brain processes of memory retrieval. Proc Natl Acad Sci U S A. 1999;96(4):1794–1799. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy W, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Friston KJ, Price CJ. Degeneracy and redundancy in cognitive anatomy. Trends in Cognitive Sciences. 2003;7:151–152. doi: 10.1016/s1364-6613(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14(11):1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gleissner U, Elger CE. The hippocampal contribution to verbal fluency in patients with temporal lobe epilepsy. Cortex. 2001;37(1):55–63. doi: 10.1016/s0010-9452(08)70557-4. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lee AT. Motion artifacts in fMRI: comparison of 2DFT with PR and spiral scan methods. Magn Reson Med. 1995;33(5):624–635. doi: 10.1002/mrm.1910330507. [DOI] [PubMed] [Google Scholar]
- Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4(11):1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, Nadel L. An fMRI study of episodic memory: retrieval of object, spatial, and temporal information. Behav Neurosci. 2004;118(5):885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44(12):2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(Pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RS. Activation of human hippocampal formation during memory for faces: a PET study. Cortex. 1995;31(1):99–108. doi: 10.1016/s0010-9452(13)80108-6. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Barense MD, Graham KS. The contribution of the human medial temporal lobe to perception: Bridging the gap between animal and human studies. The Quarterly Journal of Experimental Psychology. 2005;58B(34):300–325. doi: 10.1080/02724990444000168. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20(2):878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Luo J, Niki K. Role of medial temporal lobe in extensive retrieval of task-related knowledge. Hippocampus. 2002;12(4):487–494. doi: 10.1002/hipo.10027. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD. Recalling routes around London: activation of the right hippocampus in taxi drivers. J Neurosci. 1997;17(18):7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Rudge P, Cipolotti L. The effect of adult-acquired hippocampal damage on memory retrieval: An fMRI study. NeuroImage. 2005;27(1):146–152. doi: 10.1016/j.neuroimage.2005.04.006. ISSN: 0028-3932. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9(1):54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124(Pt 6):1156–1170. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38(1):127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Paulsen JS, Salmon DP, Brugger P, et al. A comparison of category and letter fluency in Alzheimer’s disease and Huntington’s disease. Neuropsychology. 1994;8:25–30. [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16(2):179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc Biol Sci. 1996;263(1373):989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Nadel L. Multiple memory systems: A new view. In: Byrne J, editor. Learning and Memory: A Comprehensive Reference. Elsevier Press Ltd; in press. [Google Scholar]
- Nadel L, Campbell JL, Ryan L. Autobiographical Memory Retrieval and Hippocampal Activation as a Function of Repetition and the Passage of Time. Neural Plasticity. 2007 doi: 10.1155/2007/90472. [online]. Available: http://www.hindawi.com/, Article ID 90472, 14 pages. [DOI] [PMC free article] [PubMed]
- Nadel L, Winocur G, Ryan L, Moscovitch M. Systems consolidation and hippocampus: two views. Debates in Neuroscience. 2007;1(2–4):55–66. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa S, Gilardi MC, Castiglioni I, et al. Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. NeuroReport. 1997;8:2011–2016. doi: 10.1097/00001756-199705260-00042. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Laakso M, Partanen K, et al. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol. 2000;47(4):470–476. [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends in Cognitive Sciences. 2002;6:416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Rajah MN, McIntosh AR. Overlap in the functional neural systems involved in semantic and episodic memory retrieval. J Cogn Neurosci. 2005;17(3):470–482. doi: 10.1162/0898929053279478. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Winocur G, Ziegler M, Hevenor SJ, Grady CL, Moscovitch M. fMRI studies of remote spatial memory in an amnesic person. Brain & Cognition. 2004;54(2):170–172. [PubMed] [Google Scholar]
- Ryan L, Nadel L, Keil K, Putnam K, Schnyer D, Trouard T, et al. Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11(6):707–714. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience. 2006;1(3):229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolck H, Kensinger EA, Corkin S, Squire LR. Semantic knowledge in patient H.M. and other patients with bilateral medial and lateral temporal lobe lesions. Hippocampus. 2002;12(4):520–533. doi: 10.1002/hipo.10039. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24(45):10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8(3):205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford: Clarendon Press; 1983. [Google Scholar]
- Tulving E. Episodic memory and autonoesis: Uniquely human? In: Terrace HS, Metcalfe J, editors. The Missing Link in Cognition: Origins of Self-Reflective Consciousness. Oxford University Press; 2005. pp. 3–56. [Google Scholar]
- Vallée-Tourangeau F, Anthony SH, Austin NG. Strategies for generating multiple instances of common and ad hoc categories. Memory. 1998;6(5):555–592. doi: 10.1080/741943085. [DOI] [PubMed] [Google Scholar]
- Van Overschelde JP, Rawson KA, Dunlosky J. Category norms: An updated and expanded version of the Battig and Montague (1969) norms. Journal of Memory and Language. 2004;50(3):289–335. [Google Scholar]
- Walker WH, Kintsch W. Automatic and strategic aspects of knowledge retrieval. Cognitive Science. 1985;9:261–283. [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer’s disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol. 1992;49(5):448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Mem Cognit. 2003;31(5):761–774. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]
- Williams MD, Hollan JD. The process of retrieval from very long-term memory. Cognitive Science. 1981;5:87–119. [Google Scholar]
- Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learn Mem. 2007;14(4):313–317. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Sekeres M. Memory consolidation or transformation: context manipulation and hippocampal representations of memory. Nat Neurosci. 2007;10(5):555–557. doi: 10.1038/nn1880. [DOI] [PubMed] [Google Scholar]