Abstract
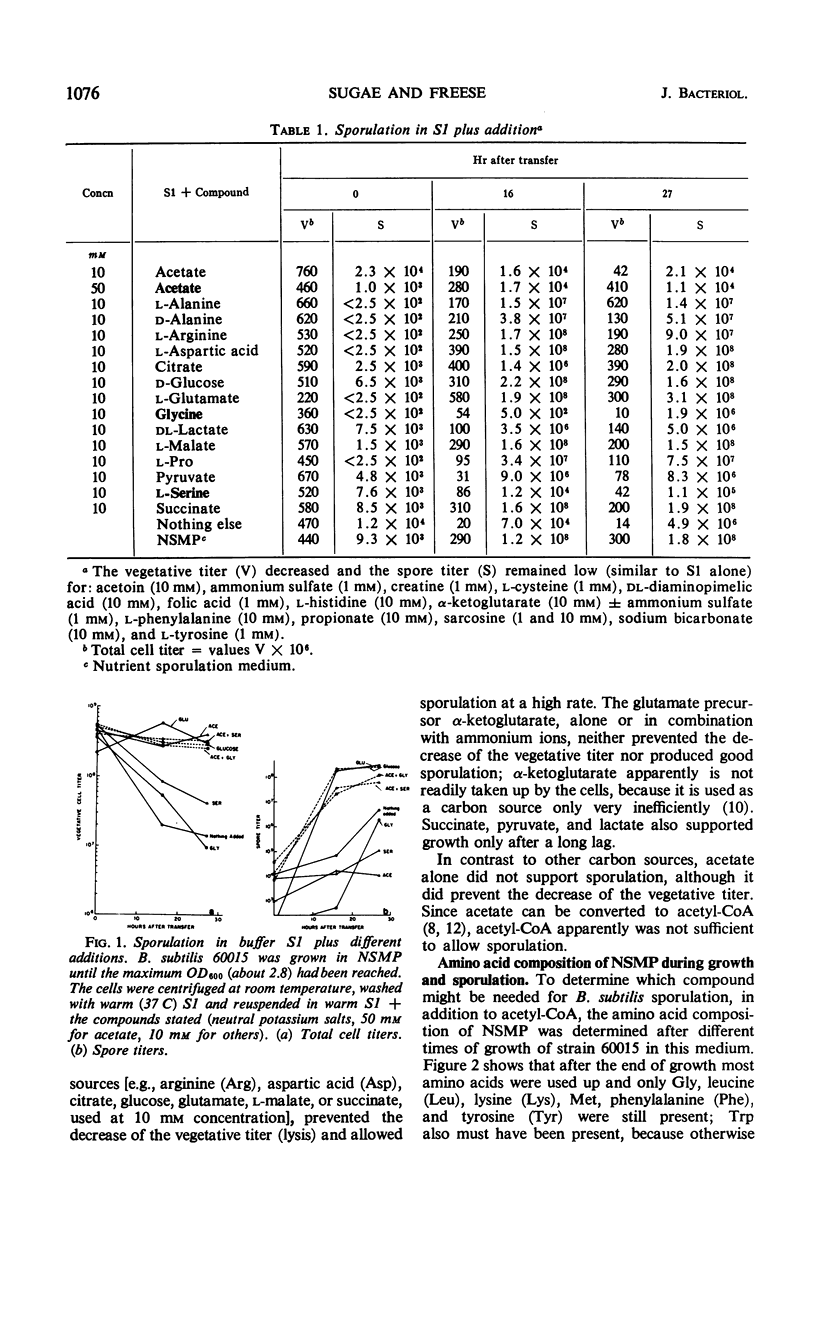
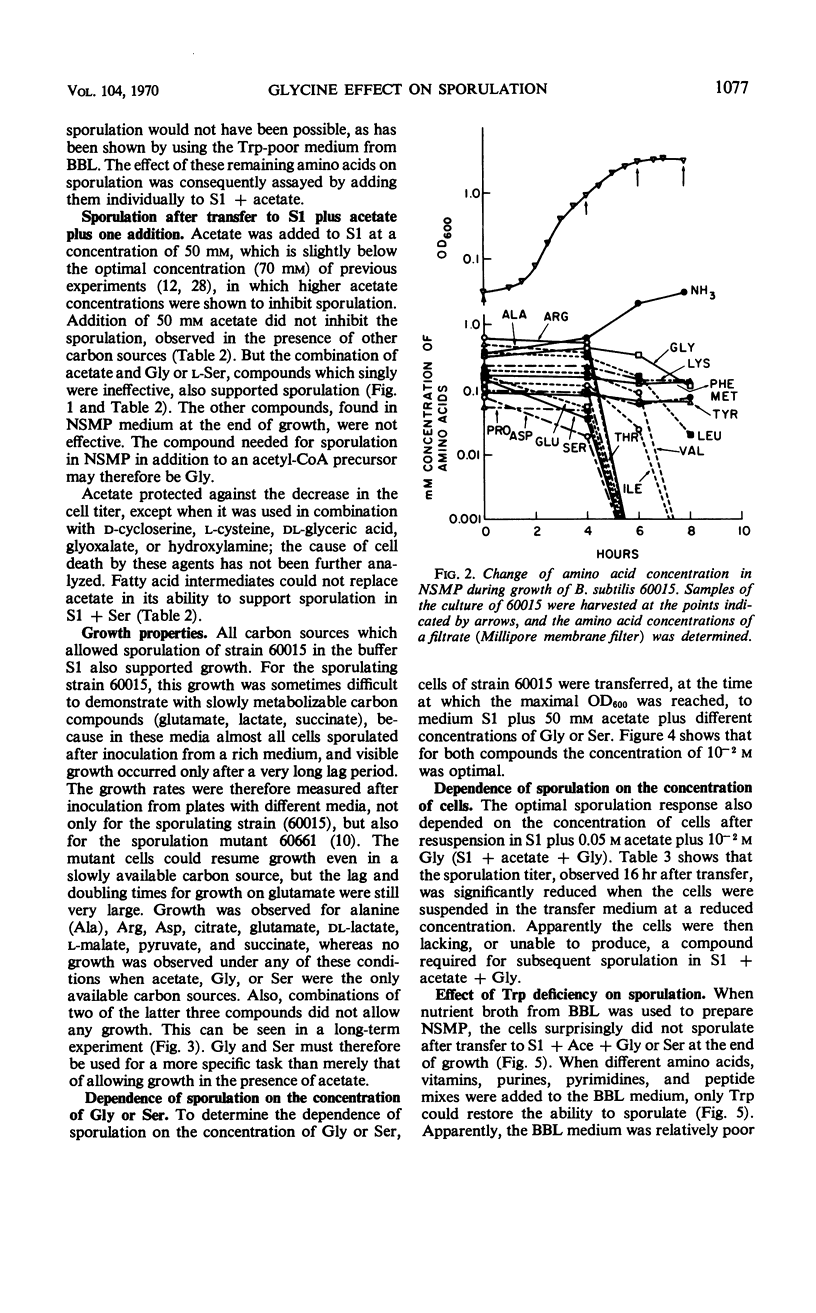
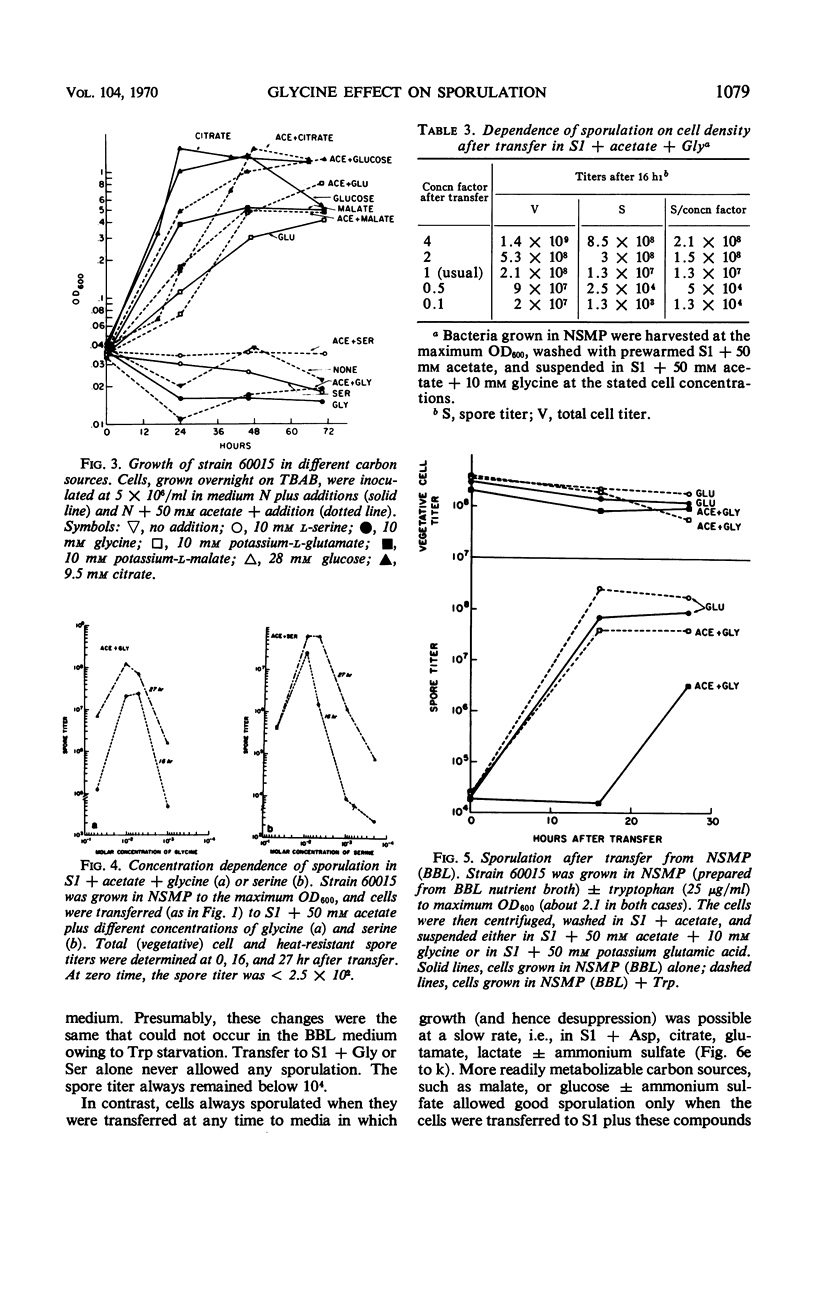
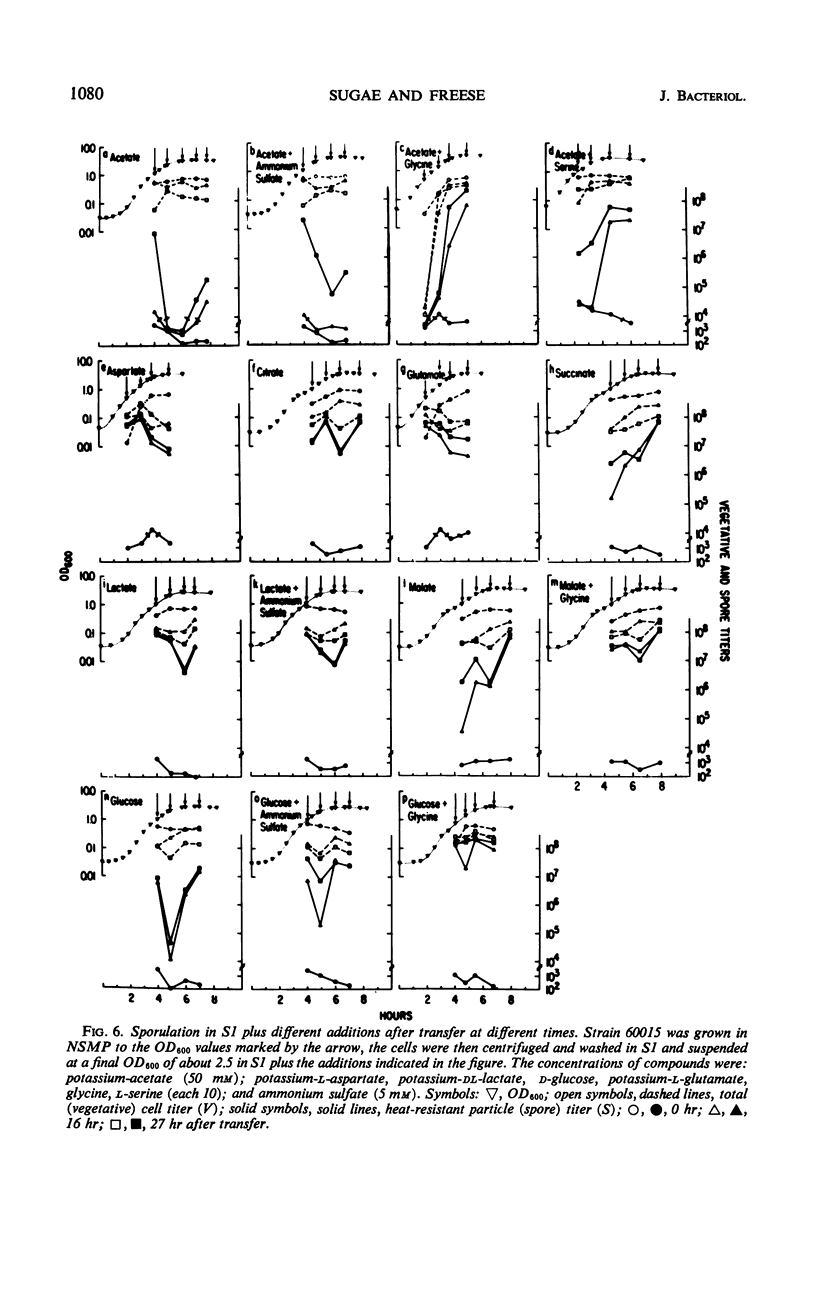
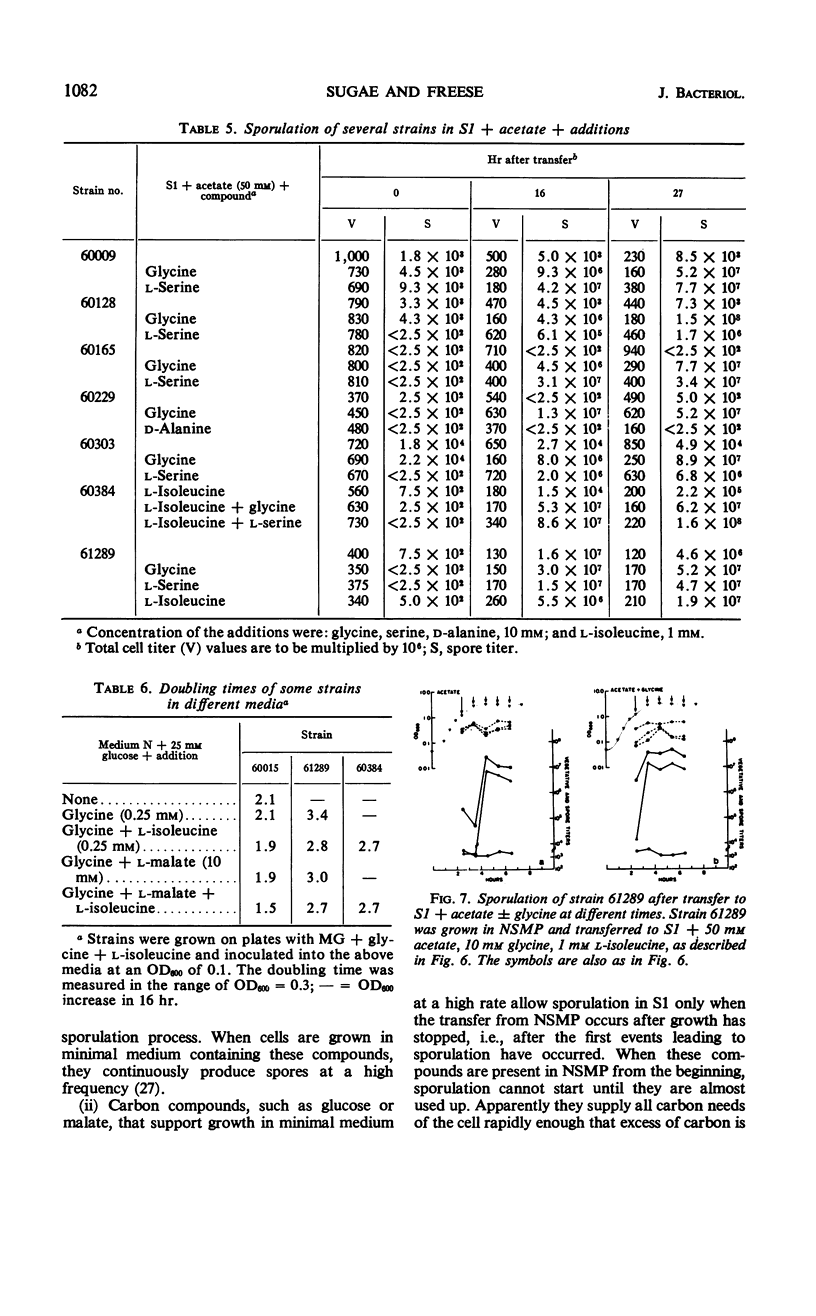
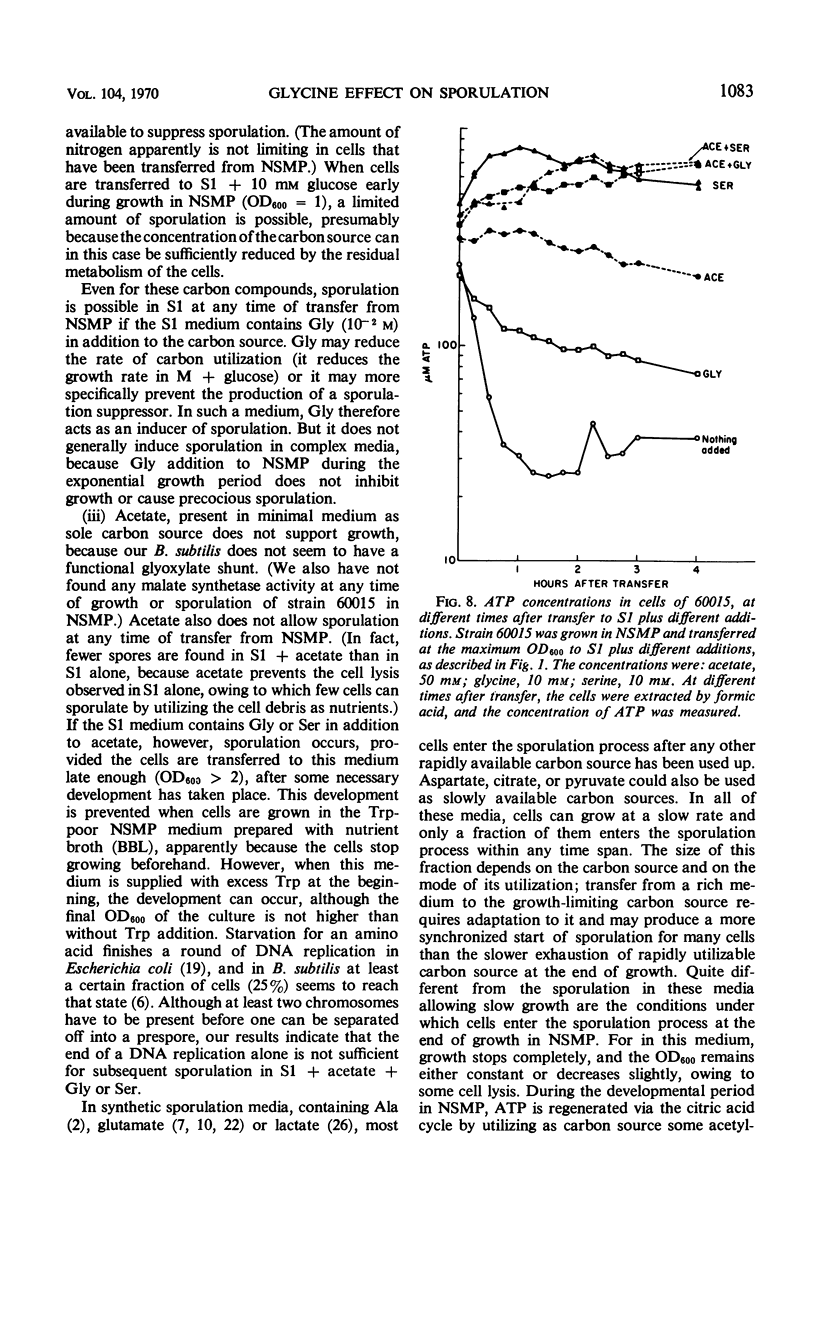
Cells of Bacillus subtilis sporulate when they are transferred, at any time of growth in nutrient sporulation medium, to a potassium-phosphate buffer containing slowly utilizable carbon sources such as l-aspartate, citrate, l-glutamate, or lactate. Transfer to buffer containing more rapidly utilizable carbon sources such as malate or glucose leads to sporulation only when the cells either had reached the end of growth or when the transfer medium also contains glycine. Acetate, which as a sole carbon source does not allow growth, also does not alone permit sporulation; however, the presence of both acetate (0.05 m) and glycine or l-serine (0.01 m) in the buffer medium allows sporulation if the cells are transferred to this medium after they have grown in the nutrient sporulation medium beyond the end of the exponential growth phase (T0). The development, required before transfer, does not seem to involve the end of a round of deoxyribonucleic acid duplication, as experiments with tryptophan-starved cells have indicated. Glycine or serine cannot be replaced by any of the known metabolites, which are partially derived from them. Amino acid analysis of nutrient sporulation medium showed that glycine (but not serine) is present at a concentration of 0.3 mm at the beginning of the developmental period, thus allowing, in combination with an acetyl-coenzyme A (CoA) precursor, sporulation but not growth. Acetyl-CoA is required not only for adenosine-triphosphate synthesis but also for some other reactions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprison M. H., Shank R. P., Davidoff R. A. A comparison of the concentration of glycine, a transmitter suspect, in different areas of the brain and spinal cord in seven different vertebrates. Comp Biochem Physiol. 1969 Mar;28(3):1345–1355. doi: 10.1016/0010-406x(69)90571-4. [DOI] [PubMed] [Google Scholar]
- BERGERE J. L., HERMIER J. ETUDE DES FACTEURS INTERVENANT DANS LA PRODUCTION MASSIVE DES SPORES DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Feb;106:214–235. [PubMed] [Google Scholar]
- Combépine G., Turian G. Métabolisme de la glycine et conidiation du Neurospora crassa. Pathol Microbiol (Basel) 1967;30(6):953–958. [PubMed] [Google Scholar]
- Copeland J. C. Regulation of chromosome replication in Bacillus subtilis: effects of amino acid starvation in strain 168. J Bacteriol. 1969 Sep;99(3):730–736. doi: 10.1128/jb.99.3.730-736.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONNELLAN J. E., Jr, NAGS E. H., LEVINSON H. S. CHEMICALLY DEFINED, SYNTHETIC MEDIA FOR SPORULATION AND FOR GERMINATION AND GROWTH OF BACILLUS SUBTILIS. J Bacteriol. 1964 Feb;87:332–336. doi: 10.1128/jb.87.2.332-336.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowell R. R. Factors controlling the sporulation of yeasts. II. The sporulation phase. J Appl Bacteriol. 1967 Dec;30(3):450–474. doi: 10.1111/j.1365-2672.1967.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel U. Growth and sporulation of Bacillus subtilis mutants blocked in the pyruvate dehydrogenase complex. J Bacteriol. 1969 Sep;99(3):745–756. doi: 10.1128/jb.99.3.745-756.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith J. C., Smith J. E. Changes in activity of certain enzymes of the tricarboxylic acid cycle and the glyoxylate cycle during the initiation of conidiation of Aspergillus niger. Can J Microbiol. 1969 Oct;15(10):1207–1212. doi: 10.1139/m69-218. [DOI] [PubMed] [Google Scholar]
- HARDWICK W. A., FOSTER J. W. On the nature of sporogenesis in some aerobic bacteria. J Gen Physiol. 1952 Jul;35(6):907–927. doi: 10.1085/jgp.35.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- Kominek L. A., Halvorson H. O. Metabolism of poly-beta-hydroxybutyrate and acetoin in Bacillus cereus. J Bacteriol. 1965 Nov;90(5):1251–1259. doi: 10.1128/jb.90.5.1251-1259.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H. M. ORGANIC NUTRIENTS REQUIRED FOR GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1964 Nov;88:1522–1524. doi: 10.1128/jb.88.5.1522-1524.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulevey-Matikian N., Turian G. Contrôle métabolique et aspects ultrastructuraux de la conidiation (macro-microconidies) de Neurospora crassa. Arch Mikrobiol. 1968;60(1):35–58. [PubMed] [Google Scholar]
- PIZER L. I. THE PATHWAY AND CONTROL OF SERINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Biol Chem. 1963 Dec;238:3934–3944. [PubMed] [Google Scholar]
- Ramaley R. F., Burden L. Replacement sporulation of Bacillus subtilis 168 in a chemically defined medium. J Bacteriol. 1970 Jan;101(1):1–8. doi: 10.1128/jb.101.1.1-8.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R., Freese E. Curing of a sporulation mutant and antibiotic activity of Bacillus subtilis. J Bacteriol. 1968 Oct;96(4):1255–1265. doi: 10.1128/jb.96.4.1255-1265.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky R. A., Law J. H. SYNTHESIS AND DEGRADATION OF POLY-beta-HYDROXYBUTYRIC ACID IN CONNECTION WITH SPORULATION OF BACILLUS MEGATERIUM. J Bacteriol. 1961 Jul;82(1):37–42. doi: 10.1128/jb.82.1.37-42.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto E., Pizer L. I. The mechanism of end product inhibition of serine biosynthesis. I. Purification and kinetics of phosphoglycerate dehydrogenase. J Biol Chem. 1968 May 10;243(9):2081–2089. [PubMed] [Google Scholar]
- TURIAN G. SYNTHETIC CONIDIOGENOUS MEDIA FOR NEUROSPORA CRASSA. Nature. 1964 Jun 20;202:1240–1240. doi: 10.1038/2021240a0. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., WILKINSON J. F. The isolation and estimation of the poly-beta-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol. 1958 Aug;19(1):198–209. doi: 10.1099/00221287-19-1-198. [DOI] [PubMed] [Google Scholar]
- Weinberger S., Gilvarg C. Bacterial distribution of the use of succinyl and acetyl blocking groups in diaminopimelic acid biosynthesis. J Bacteriol. 1970 Jan;101(1):323–324. doi: 10.1128/jb.101.1.323-324.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



