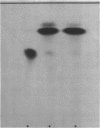
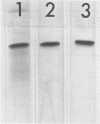
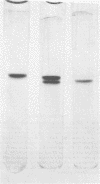
Abstract
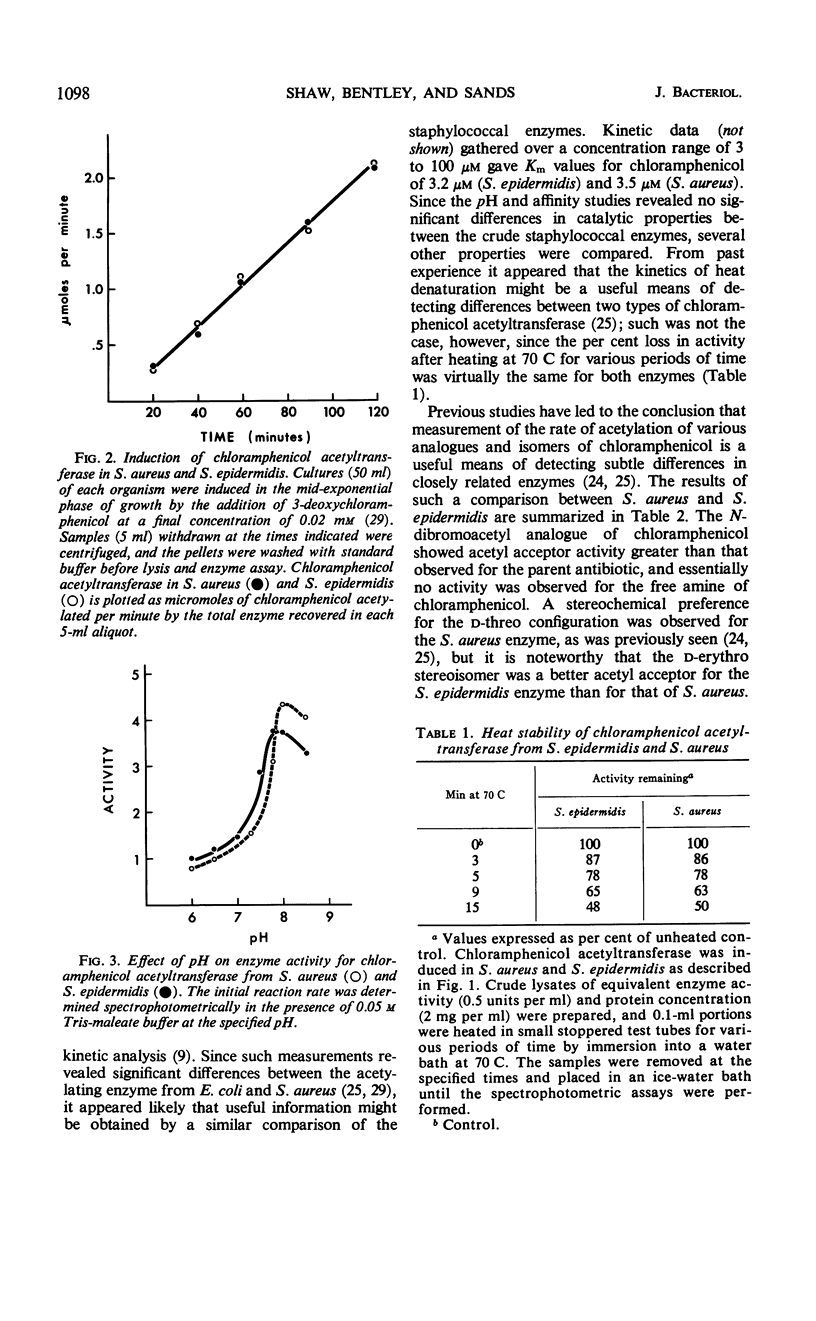
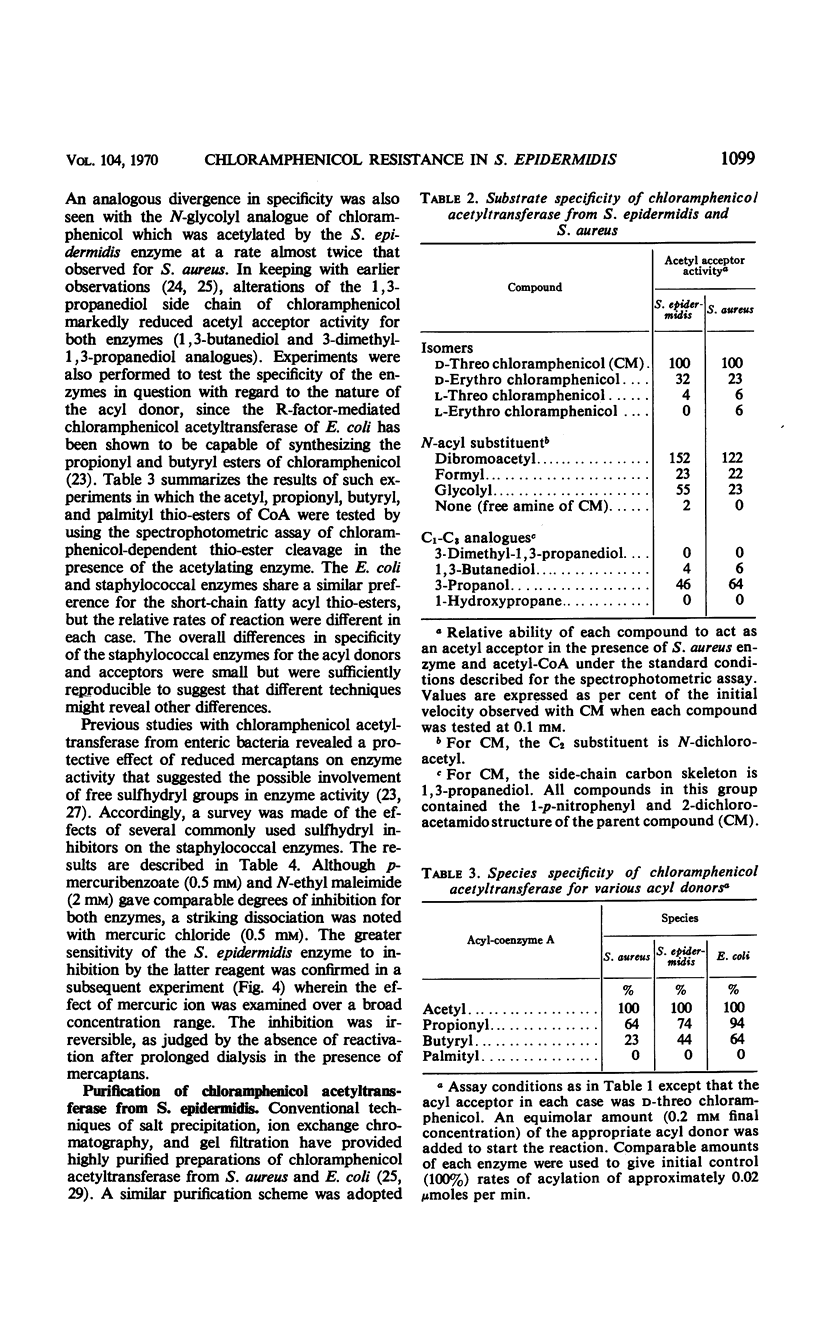
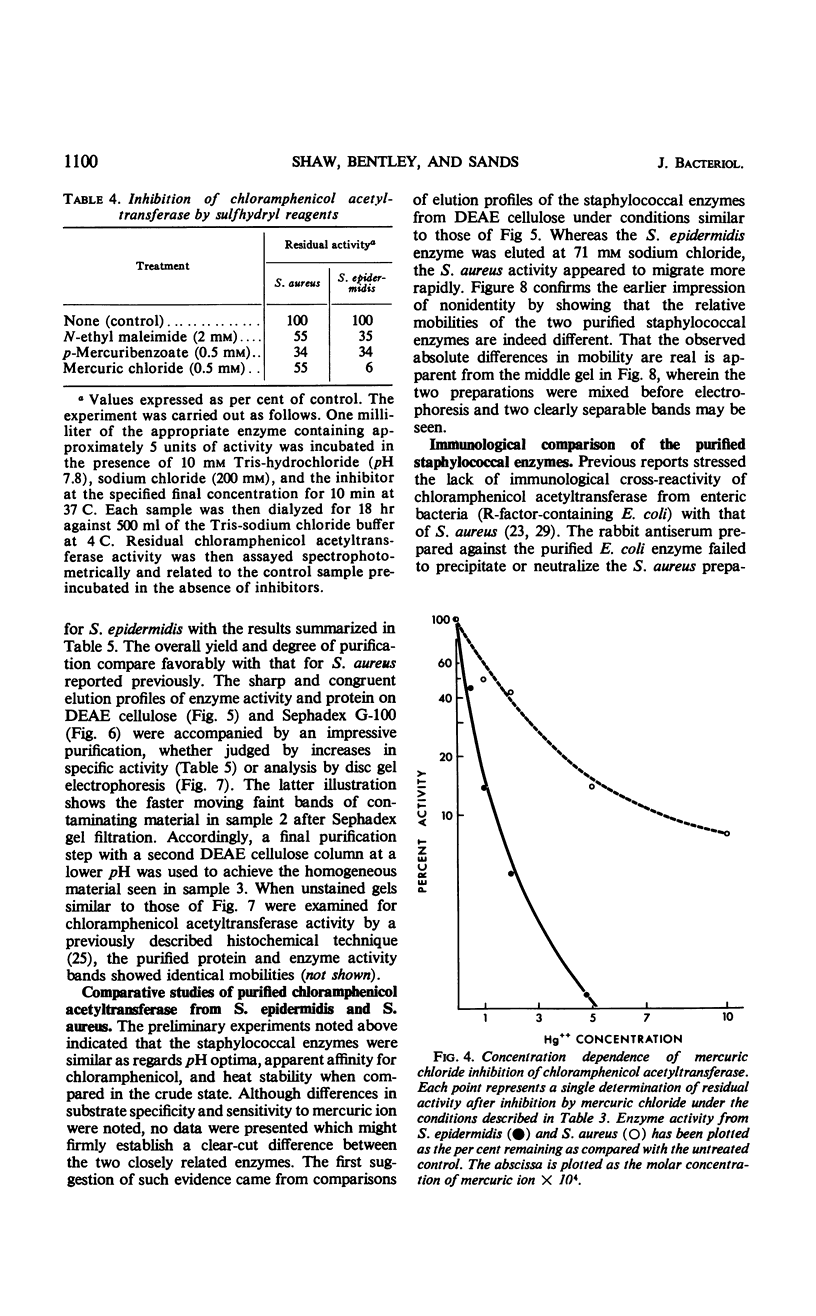
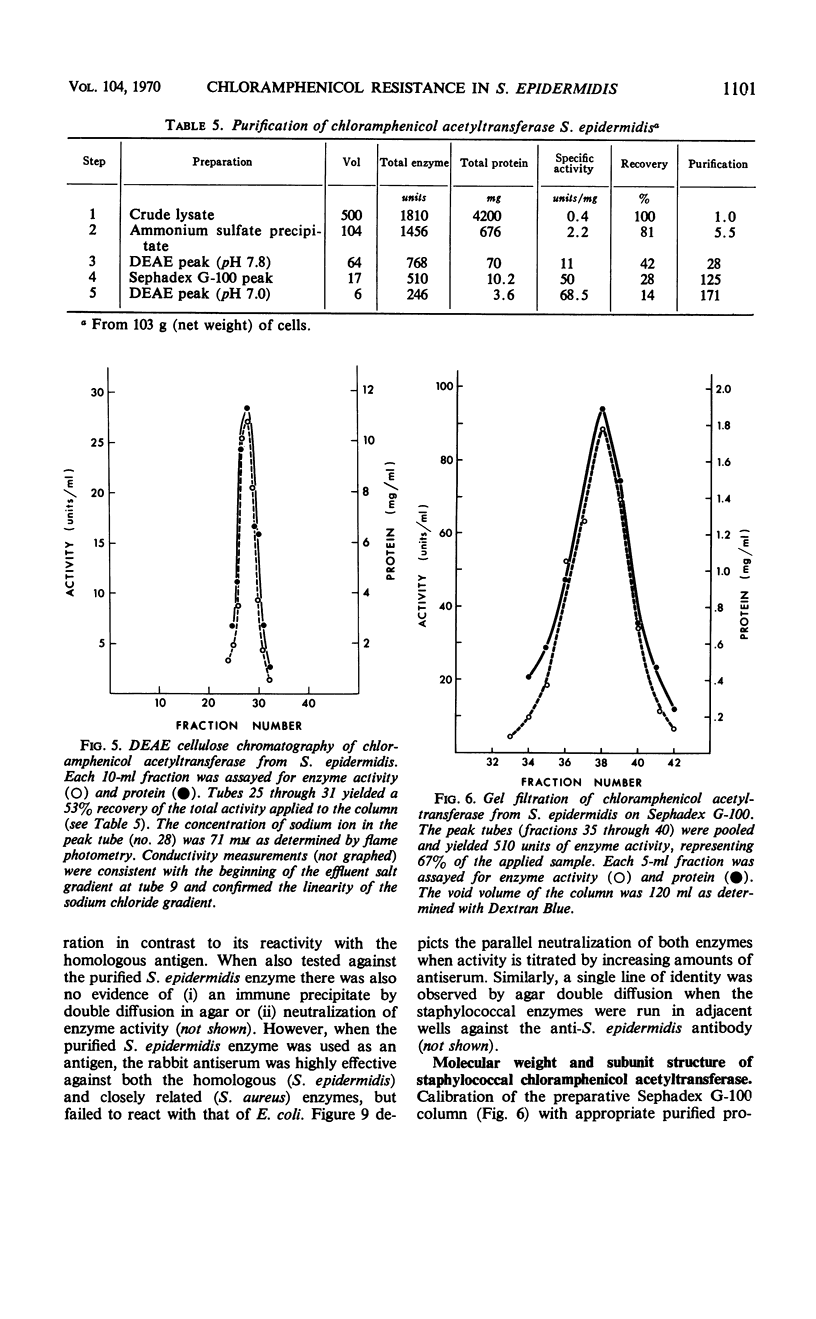
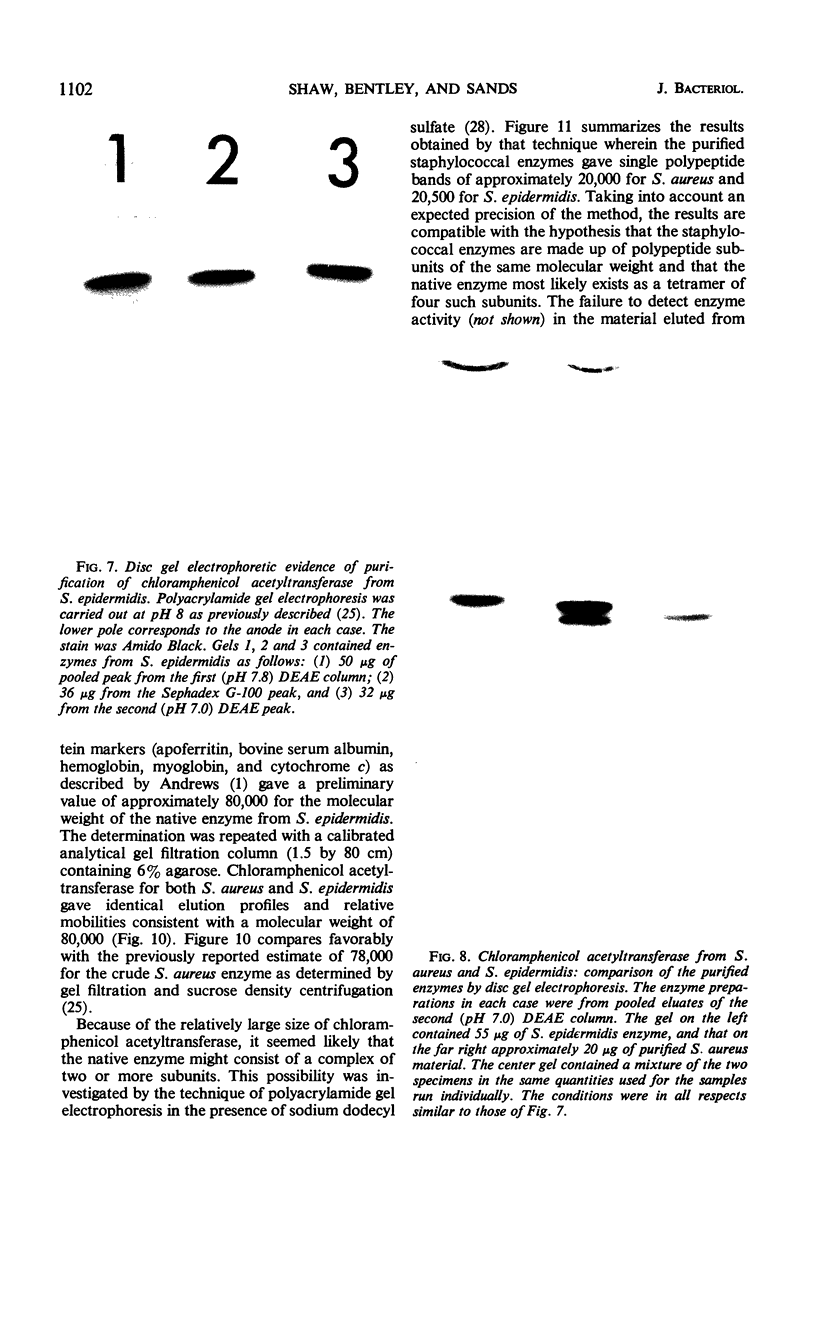
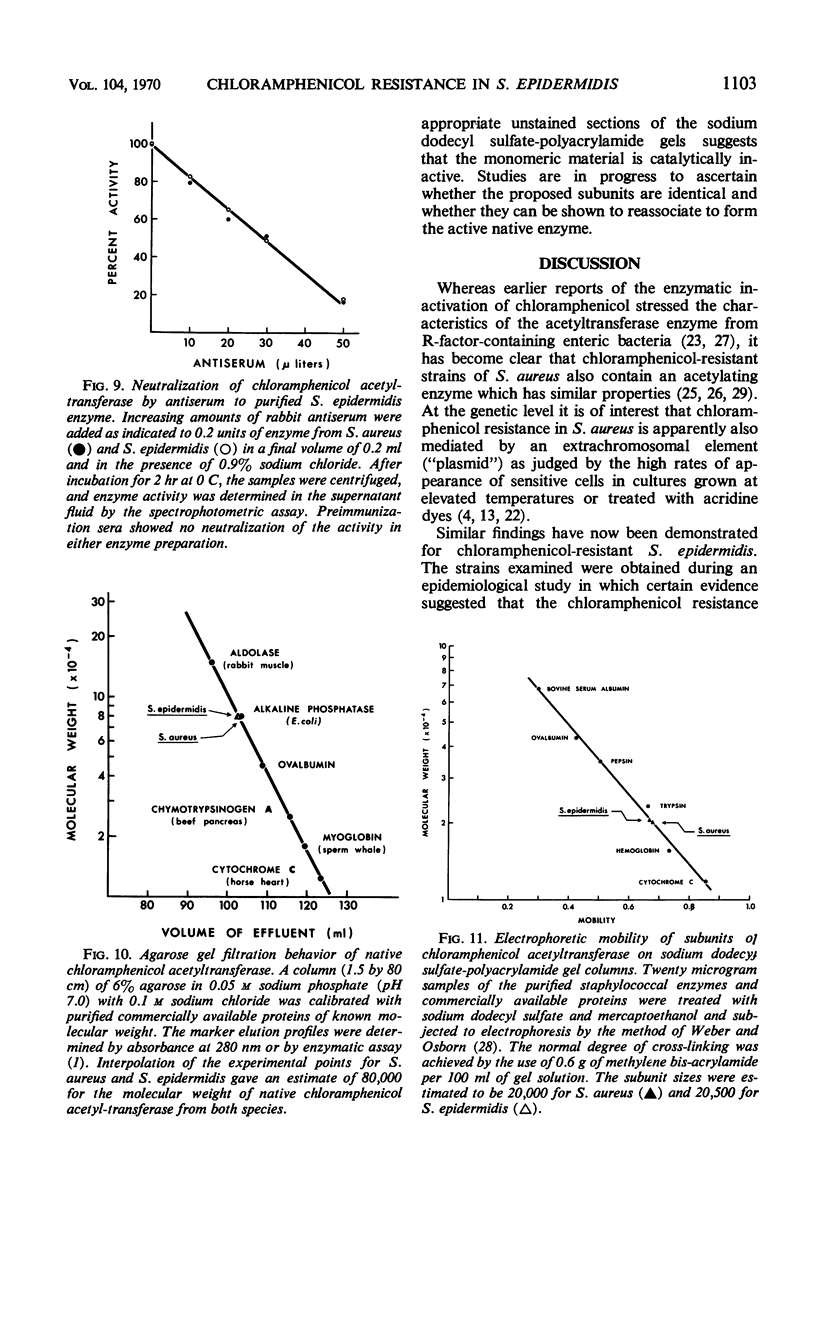
The mechanism of chloramphenicol resistance in several multiple-resistant Staphylococcus epidermidis strains has been studied and shown to be due to the presence of the enzyme, chloramphenicol acetyltransferase. As with S. aureus, the inactivating enzyme in S. epidermidis appears to be the product of a structural gene on the chloramphenicol plasmid because resistance and enzyme activity are concurcurrently lost after growth in acridine orange or at elevated temperatures. The synthesis of chloramphenicol acetyltransferase in S. epidermidis has been compared with the function of a similar enzyme in chloramphenicol-resistant S. aureus with the conclusion that the kinetics of induction, products of the reaction, and general properties of the enzymes are identical. The chloramphenicol acetylating enzyme from S. epidermidis has been purified to a state of homogeneity and compared with the analogous purified S. aureus enzyme. Both purified preparations consist of native enzymes with molecular weights of 80,000, and evidence is presented that is consistent with their being made up of four identical subunits of 20,000 each. The two staphylococcal enzymes are identical with respect to pH optimum, apparent affinity (Km) for chloramphenicol, heat denaturation, and immunological reactivity, but they differ in electrophoretic mobility, chromatographic behavior, substrate specificity, and sensitivity to inhibition by mercuric ion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIRD-PARKER A. C. THE CLASSIFICATION OF STAPHYLOCOCCI AND MICROCOCCI FROM WORLD-WIDE SOURCES. J Gen Microbiol. 1965 Mar;38:363–387. doi: 10.1099/00221287-38-3-363. [DOI] [PubMed] [Google Scholar]
- Baldwin J. N., Strickland R. H., Cox M. F. Some properties of the beta-lactamase genes in Staphylococcus epidermidis. Appl Microbiol. 1969 Oct;18(4):628–630. doi: 10.1128/am.18.4.628-630.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABBERT Y. A., BAUDENS J. G., GERBAUD G. R. VARIATIONS SOUS L'INFLUENCE DE L'ACRIFLAVINE ET TRANSDUCTION DE LA R'ESISTANCE A LA KANAMYCINE ET AU CHLORAMPH'ENICOL CHEZ LES STAPHYLOCOQUES. Ann Inst Pasteur (Paris) 1964 Nov;107:678–690. [PubMed] [Google Scholar]
- Corse J., Williams R. E. Antibiotic resistance of coagulase-negative staphylococci and micrococci. J Clin Pathol. 1968 Nov;21(6):722–728. doi: 10.1136/jcp.21.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S., Niwa C., Kuwahara S. Transduction of drug-resistances in Staphylococcus. II. Transduction of chloramphenicol-resistance in both Staphylococcus aureus and Staphylococcus epidermidis by typing phage 80. Jpn J Microbiol. 1965 Mar;9(1):15–19. [PubMed] [Google Scholar]
- Kono M., Ogawa K., Mitsuhashi S. Drug resistance of staphylococci. VI. Genetic determinant for chloramphenicol resistance. J Bacteriol. 1968 Mar;95(3):886–892. doi: 10.1128/jb.95.3.886-892.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., MORIMURA M., KONO K., OSHIMA H. ELIMINATION OF DRUG RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1963 Jul;86:162–164. doi: 10.1128/jb.86.1.162-164.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAMURA S. INACTIVATION OF CHLORAMPHENICOL BY CHLORAMPHENICOL-RESISTANT BACTERIA. J Pharm Sci. 1964 Jun;53:604–607. doi: 10.1002/jps.2600530606. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. Associated diploids involving penicillinase plasmids in Staphylococcus aureus. J Gen Microbiol. 1967 Jan;46(1):85–93. doi: 10.1099/00221287-46-1-85. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. New type of restriction to the expression of a structural gene in bacteria. Nature. 1967 Dec 23;216(5121):1191–1192. doi: 10.1038/2161191a0. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S., Kono M. Basis of chloramphenicol resistance in naturally isolated resistant staphylococci. J Bacteriol. 1966 Sep;92(3):798–799. doi: 10.1128/jb.92.3.798-799.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S. The enzymatic acetylation of chloramphenicol by the multiple drug-resistant Escherichia coli carrying R factor. J Biol Chem. 1967 Oct 25;242(20):4722–4730. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Winshell E., Shaw W. V. Kinetics of induction and purification of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1969 Jun;98(3):1248–1257. doi: 10.1128/jb.98.3.1248-1257.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]