Abstract
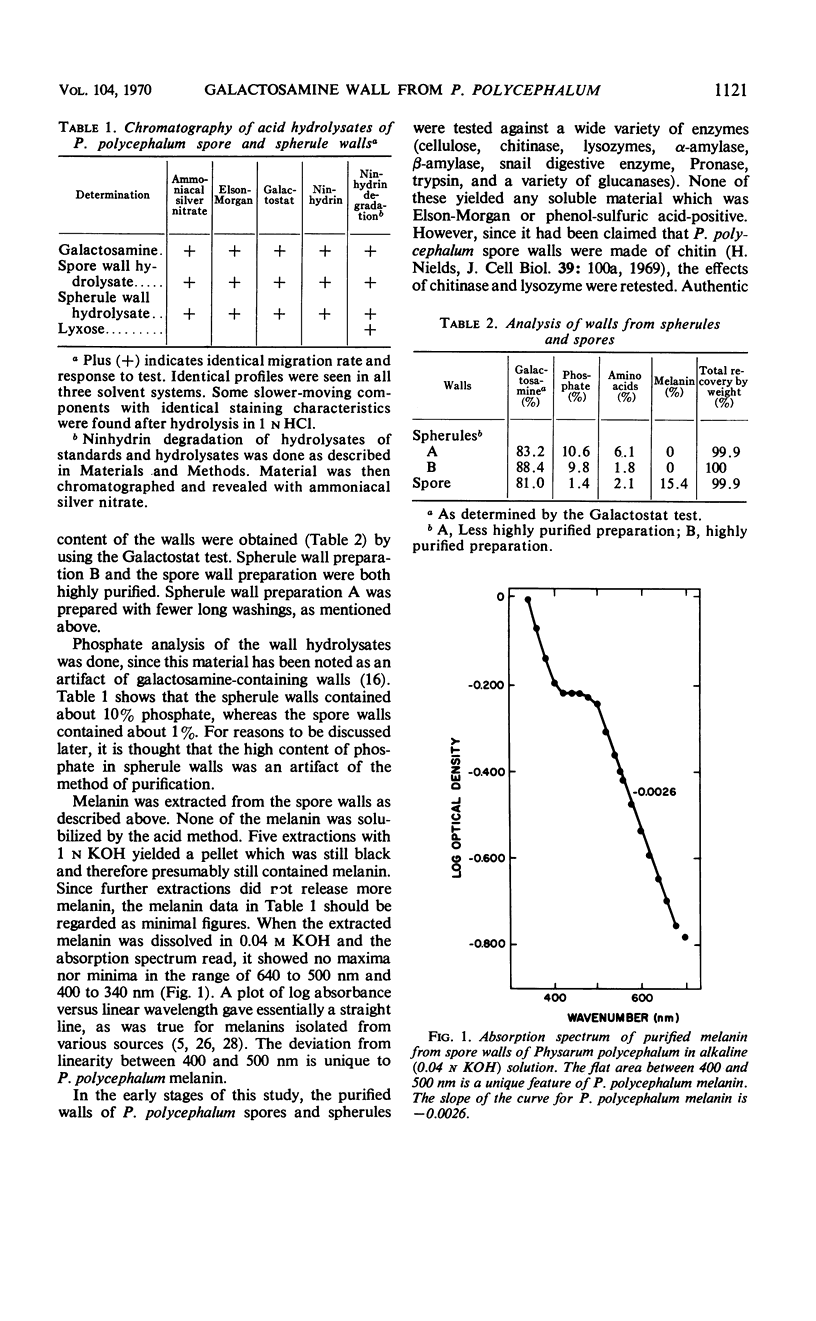
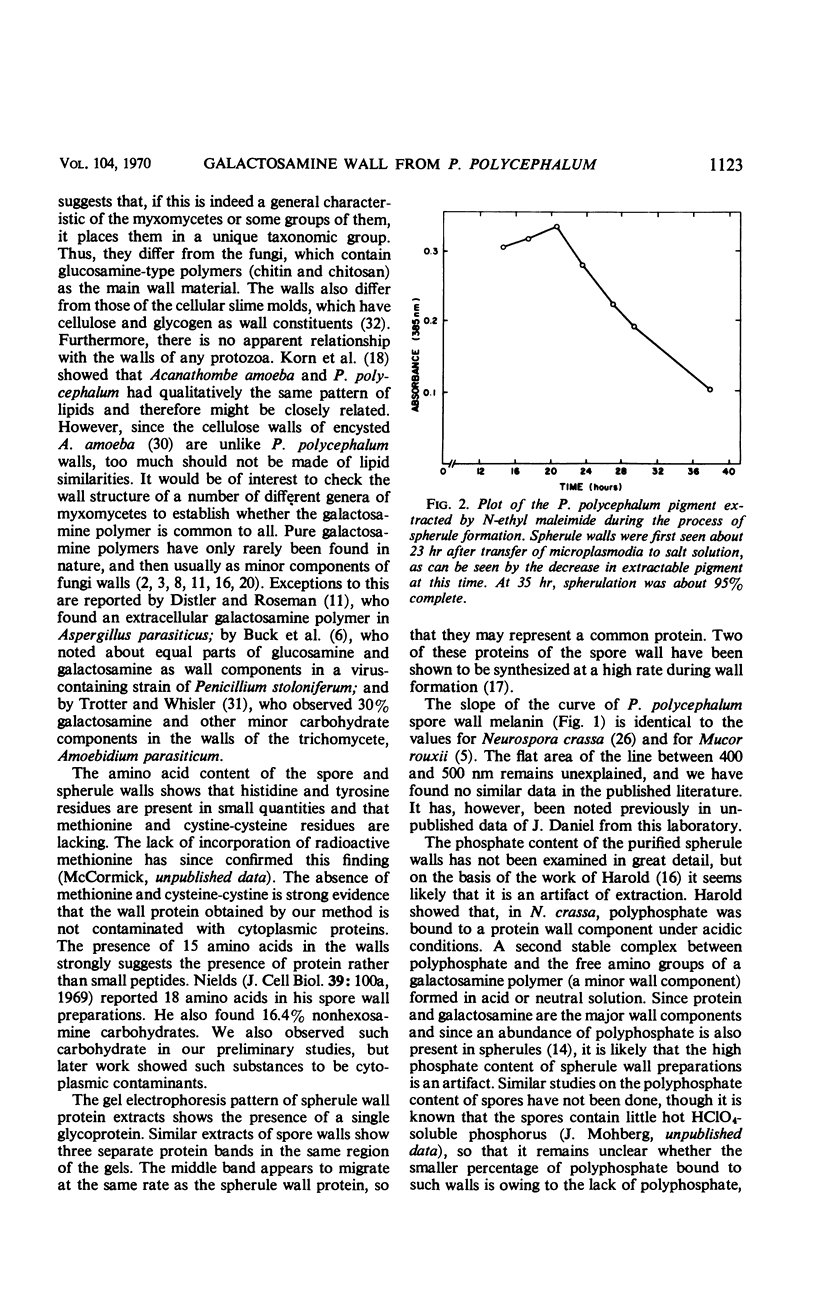
The myxomycete, Physarum polycephalum, can be induced under laboratory conditions to form two different hard-walled forms, spores and spherules. Characterization of both types of walls revealed only a single sugar, galactosamine. It was identified after acid hydrolysis of the isolated walls by chromatography in three solvent systems, by its positive reaction with ammoniacal silver nitrate, ninhydrin, Galactostat, and the Elson-Morgan test, and by ninhydrin degradation to lyxose. Galactosamine was present as a polymer with solubility characteristics the same as the β1-4–linked glucosamine polymer (chitosan). The walls were also found to contain about 2% protein. Spherule walls revealed a single glycoprotein on gel electrophoresis. Spore walls contained a similar protein component. The phosphate content of isolated spherule walls was 9.8%, and that of spore walls was 1.4%. Spore walls also contained about 15% melanin which was shown to be similar to fungal melanin. A novel method was used to measure the rate of mature spherule formation based on the loss of extractability of P. polycephalum natural pigment. The presence of a rare galactosamine polymer in P. polycephalum spore and spherule walls as the only carbohydrate suggests that the myxomycetes are not closely related to the fungi or the protozoa.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. J., Smith Q. T. Application of a rapid and reliable method for determination of hexosamine in the skin of estrogen-treated rats. Anal Biochem. 1965 Dec;13(3):510–517. doi: 10.1016/0003-2697(65)90345-3. [DOI] [PubMed] [Google Scholar]
- Applegarth D. A., Bozoian G. Presence of chitin in the cell wall of a griseofulvin-producing species of penicillium. J Bacteriol. 1967 Nov;94(5):1787–1788. doi: 10.1128/jb.94.5.1787-1788.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., REYES E. CHEMISTRY OF SPORE WALL DIFFERENTIATION IN MUCOR ROUXII. Arch Biochem Biophys. 1964 Oct;108:125–133. doi: 10.1016/0003-9861(64)90363-7. [DOI] [PubMed] [Google Scholar]
- Buck K. W., Chain E. B., Darbyshire J. E. High cell wall galactosamine content and virus particles in Penicillium stoloniferum. Nature. 1969 Sep 20;223(5212):1273–1273. doi: 10.1038/2231273a0. [DOI] [PubMed] [Google Scholar]
- CROOK E. M., JOHNSTON I. R. The qualitative analysis of the cell walls of selected species of fungi. Biochem J. 1962 May;83:325–331. doi: 10.1042/bj0830325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISTLER J. J., ROSEMAN S. Galactosamine polymers produced by Aspergillus parasiticus. J Biol Chem. 1960 Sep;235:2538–2541. [PubMed] [Google Scholar]
- Delachambre J. La réaction de la chitine à l'acide-periodique-schiff. Histochemie. 1969;20(1):58–67. doi: 10.1007/BF00402524. [DOI] [PubMed] [Google Scholar]
- Goodman E. M., Rusch H. P. Ultrastructural changes during spherule formation in Physarum polycephalum. J Ultrastruct Res. 1970 Jan;30(1):172–183. doi: 10.1016/s0022-5320(70)90071-7. [DOI] [PubMed] [Google Scholar]
- Goodman E. M., Sauer H. W., Sauer L., Rusch H. P. Polyphosphate and other phosphorus compounds during growth and differentiation of Physarum polycephalum. Can J Microbiol. 1969 Nov;15(11):1325–1331. doi: 10.1139/m69-240. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. Binding of inorganic polyphosphate to the cell wall of Neurospora crassa. Biochim Biophys Acta. 1962 Feb 12;57:59–66. doi: 10.1016/0006-3002(62)91077-6. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Sauer H. W., Brown D. F., Babcock K. L., Rusch H. P. Differential protein synthesis during sporulation in the slime mold Physarum polycephalum. J Bacteriol. 1970 Aug;103(2):356–363. doi: 10.1128/jb.103.2.356-363.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D., GREENBLATT C. L., LEES A. M. SYNTHESIS OF UNSATURATED FATTY ACIDS IN THE SLIME MOLD PHYSARUM POLYCEPHALUM AND THE ZOOFLAGELLATES LEISHMANIA TARENTOLAE, TRYPANOSOMA LEWISI, AND CRITHIDIA SP.: A COMPARATIVE STUDY. J Lipid Res. 1965 Jan;6:43–50. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Relationship of the major constituents of the Neurospora crassa cell wall to wild-type and colonial morphology. J Bacteriol. 1965 Oct;90(4):1073–1081. doi: 10.1128/jb.90.4.1073-1081.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. J., Blomquist J. C., Rusch H. P. Isolation and Characterization of an Extracellular Polysaccharide from Physarum polycephalum. J Bacteriol. 1970 Dec;104(3):1110–1118. doi: 10.1128/jb.104.3.1110-1118.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnett T., Derrenbacker E. C. The amino acid composition of algal cell walls. J Gen Microbiol. 1966 Jul;44(1):105–114. doi: 10.1099/00221287-44-1-105. [DOI] [PubMed] [Google Scholar]
- Rusch H. P. Some biochemical events in the growth cycles of Physarum polycephalum. Fed Proc. 1969 Nov-Dec;28(6):1761–1770. [PubMed] [Google Scholar]
- SCHAEFFER P. A black mutant of Neurospora crassa; mode of action of the mutant allele and action of light on melanogenesis. Arch Biochem Biophys. 1953 Dec;47(2):359–379. doi: 10.1016/0003-9861(53)90473-1. [DOI] [PubMed] [Google Scholar]
- STEIN W. D. A comparison of some natural and synthetic melanins. Nature. 1954 Sep 25;174(4430):601–602. doi: 10.1038/174601a0. [DOI] [PubMed] [Google Scholar]
- STOFFYN P. J., JEANLOZ R. W. Identification of amino sugars by paper chromatography. Arch Biochem Biophys. 1954 Oct;52(2):373–379. doi: 10.1016/0003-9861(54)90137-x. [DOI] [PubMed] [Google Scholar]
- Sauer H. W., Babcock K. L., Rusch H. P. Sporulation in Physarum polycephalum: a model system for studies on differentiation. Exp Cell Res. 1969 Oct;57(2):319–327. doi: 10.1016/0014-4827(69)90156-6. [DOI] [PubMed] [Google Scholar]
- Sempere J. M., Gancedo C., Asensio C. Determination of galactosamine and N-acetylgalactosamine in the presence of other hexosamines with galactose oxidase. Anal Biochem. 1965 Sep;12(3):509–515. doi: 10.1016/0003-2697(65)90217-4. [DOI] [PubMed] [Google Scholar]
- TOMLINSON G., JONES E. A. Isolation of cellulose from the cyst wall of a soil amoeba. Biochim Biophys Acta. 1962 Sep 10;63:194–200. doi: 10.1016/0006-3002(62)90353-0. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]



