Abstract
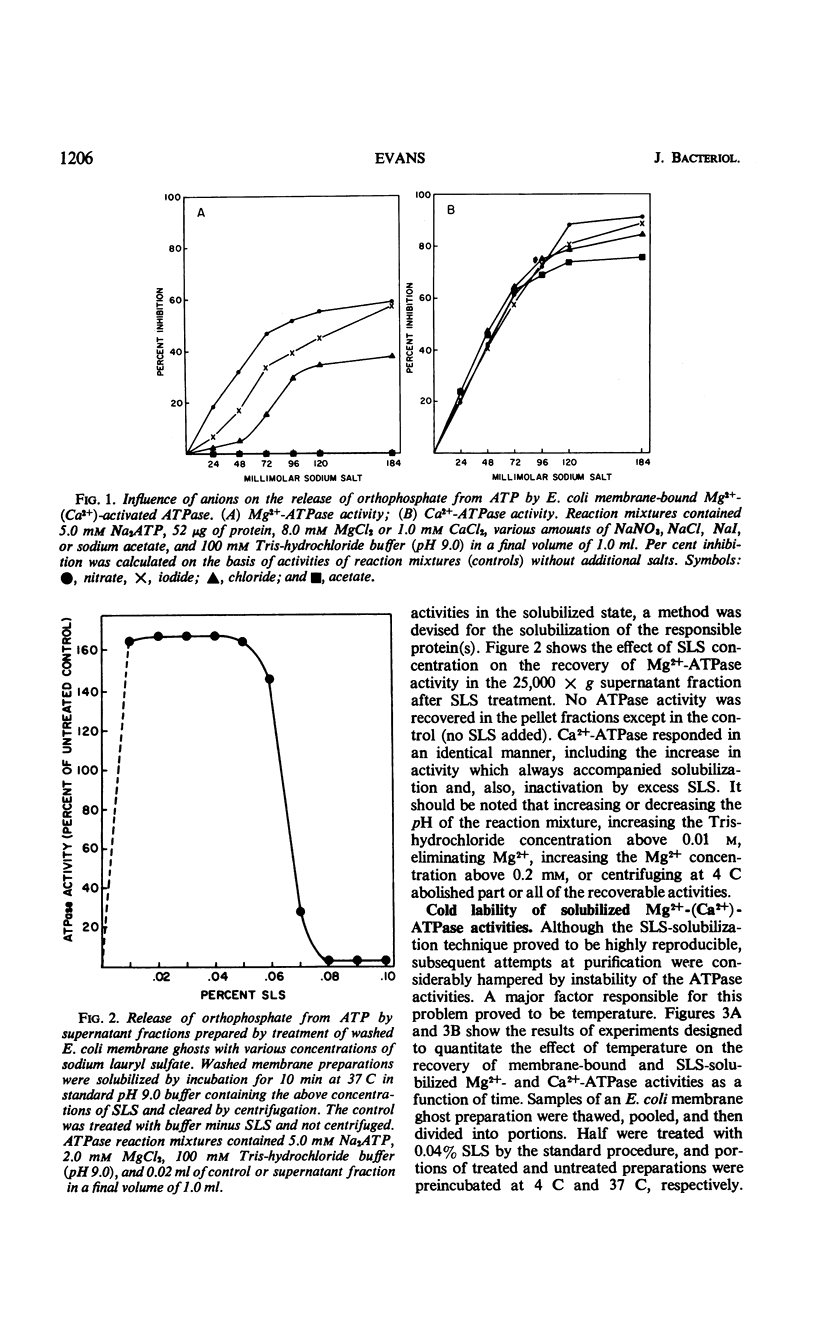
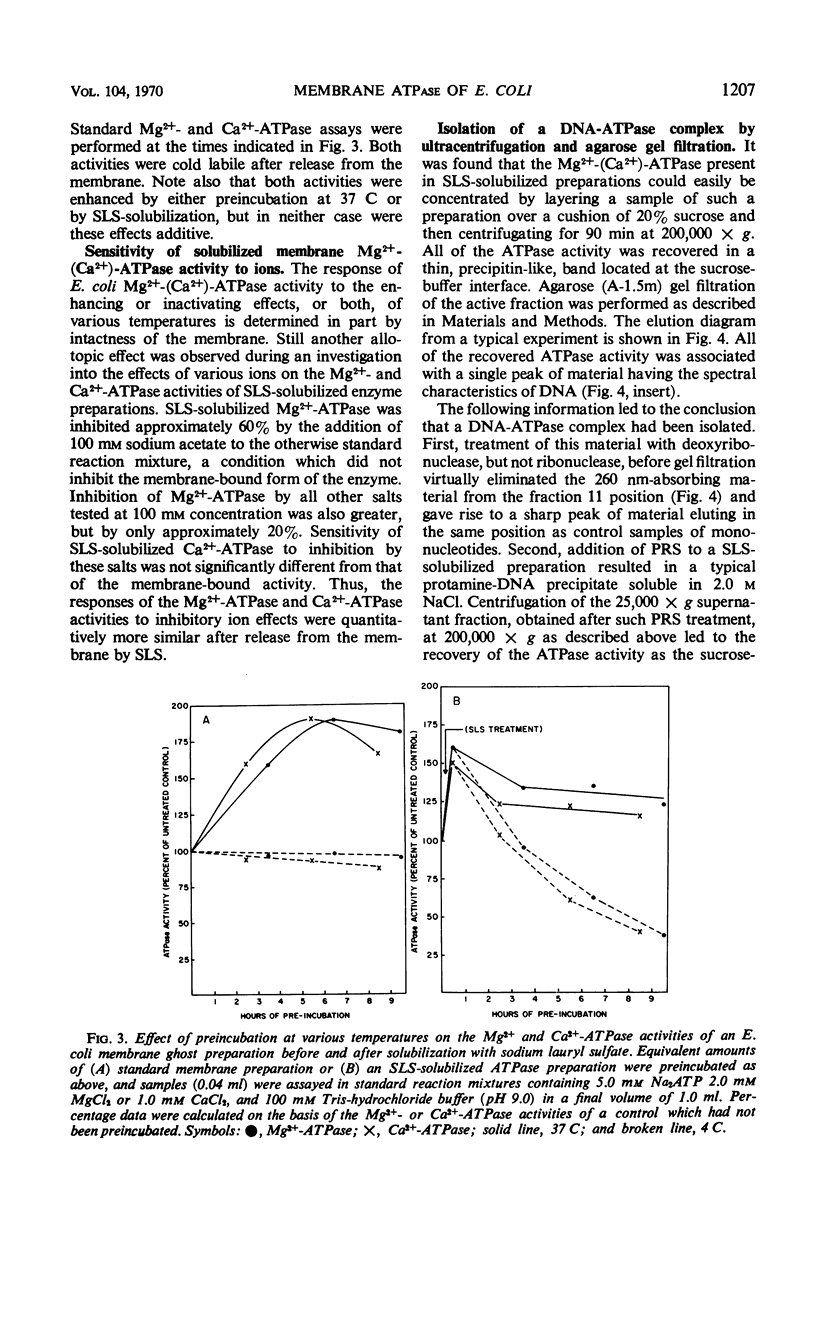
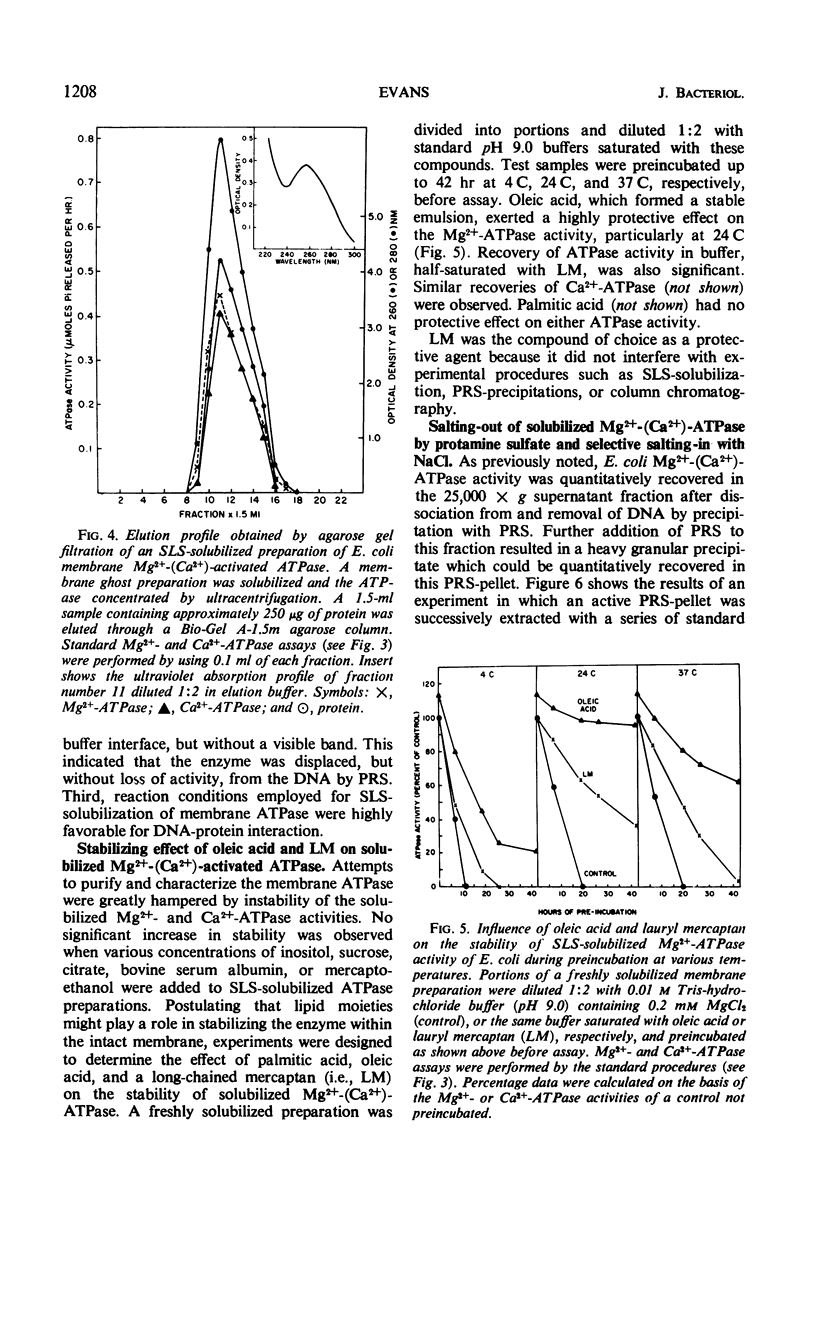
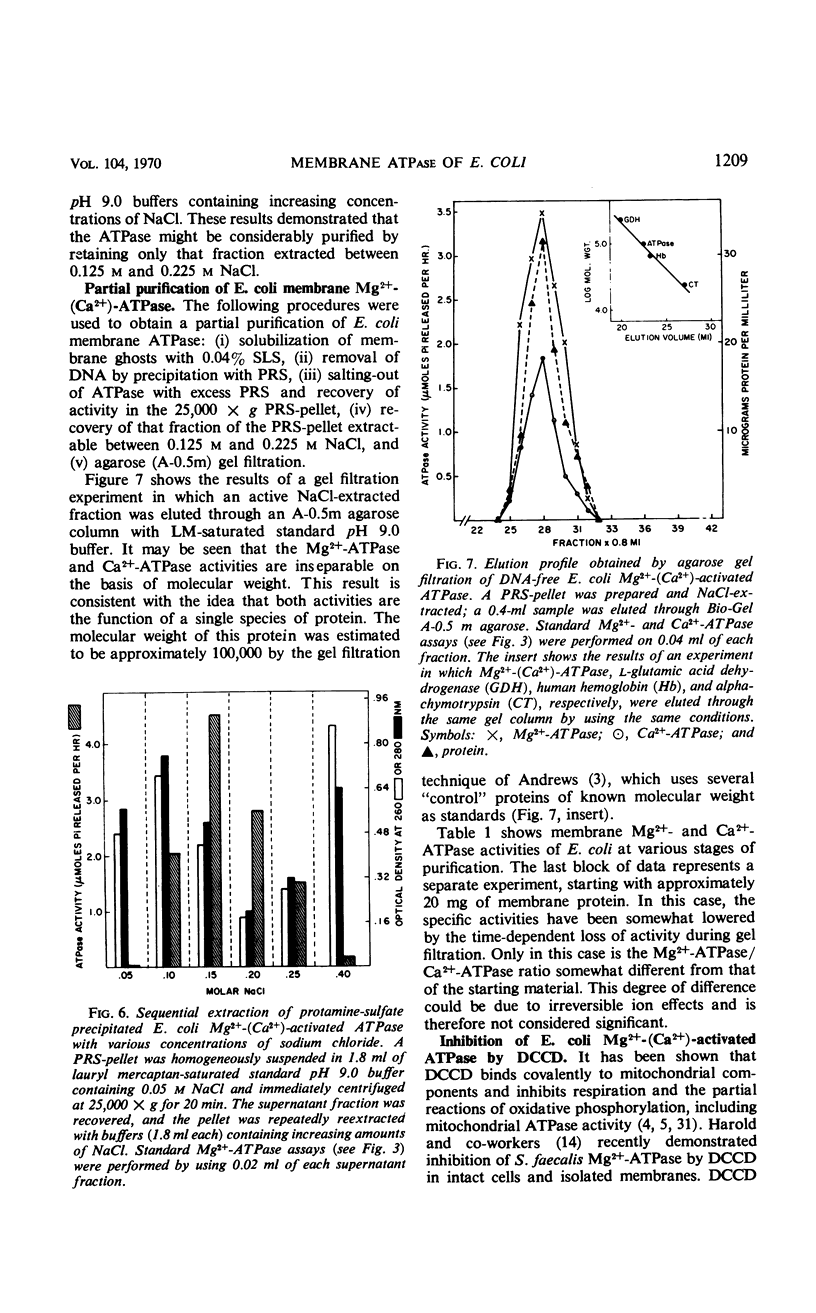
The membrane-associated Mg2+-activated and Ca2+-activated adenosine 5′-triphosphatase (EC 3.6.1.3; ATPase) activities of Escherichia coli were further characterized. The degree of inhibition of membrane-bound Mg2+-(Ca2+)-ATPase by a series of anions (i.e., sodium salts of nitrate, iodide, chloride, and acetate) was found to correlate with the relative chaotropic, or solubilizing, effectiveness of these anions. The enzyme was solubilized from washed membrane ghosts by treatment with 0.04% sodium lauryl sulfate at pH 9.0 and 37 C. Solubilized Mg2+-(Ca2+)-ATPase exhibited an initial increase in activity, followed by fairly rapid inactivation, both ATPase activities being particularly cold-labile. The combined stabilizing effects of lauryl mercaptan (1-dodecanethiol), 0.01 m tris(hydroxymethyl)amino-methane-hydrochloride buffer (pH 9.0), 0.2 mm MgCl2, and ambient temperature facilitated partial purification of the enzyme, the molecular weight of which was estimated to be approximately 100,000 by the gel filtration technique. In general, the membrane-associated Mg2+-(Ca2+)-ATPase of E. coli resembles both mitochondrial membrane ATPase and the well-characterized membrane ATPases of Bacillus megaterium and Microcococcus lysodeikticus. It is of particular interest that N,N′-dicyclohexylcarbodiimide (DCCD), a known inhibitor of mitochondrial ATPase, of mitochondrial oxidative phosphorylation, and of the membrane-bound Mg2+-ATPase of Streptococcus faecalis was found to inhibit both the membrane-bound and the solubilized forms of E. coli Mg2+-(Ca2+)-ATPase. The sensitivity of the membrane-associated Mg2+-(Ca2+)-ATPase of E. coli to both anions and cations, its allotopic behavior, and its susceptibility to inhibition by DCCD favor the idea that this enzyme plays a key, probably polyfunctional, role in such biological activities of the membrane as oxidative phosphorylation and ion transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER P. The combination of protamine with desoxyribonucleic acid. Biochim Biophys Acta. 1953 Apr;10(4):595–599. doi: 10.1016/0006-3002(53)90302-3. [DOI] [PubMed] [Google Scholar]
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Bulos B., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XVII. Further resolution of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1968 Jul 25;243(14):3891–3900. [PubMed] [Google Scholar]
- Cole H. A., Hughes D. E. The enzymic activity of the outer shell of Lactobacillus arabinosus. J Gen Microbiol. 1965 Jul;40(1):81–95. doi: 10.1099/00221287-40-1-81. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr Membrane adenosine triphosphatase of Escherichia coli: activation by calcium ion and inhibition by monovalent cations. J Bacteriol. 1969 Nov;100(2):914–922. doi: 10.1128/jb.100.2.914-922.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P., DOTY P. A light scattering investigation of the interaction of sodium desoxyribonucleate with bovine serum albumin. Biochim Biophys Acta. 1952 Dec;9(6):609–618. doi: 10.1016/0006-3002(52)90221-7. [DOI] [PubMed] [Google Scholar]
- Gross R., Coles N. W. Adenosine triphosphatase in isolated membranes of Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1322–1326. doi: 10.1128/jb.95.4.1322-1326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. SOLUBILITY AND COMPOSITION OF PROTEIN-DEOXYRIBONUCLEIC ACID COMPLEXES. Biochim Biophys Acta. 1964 Oct 16;91:340–343. doi: 10.1016/0926-6550(64)90263-4. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Soluble complexes of nucleic acids with alpha-chymotrypsin and its derivatives. Biochim Biophys Acta. 1962 Apr 2;55:440–454. doi: 10.1016/0006-3002(62)90977-0. [DOI] [PubMed] [Google Scholar]
- Hafkenscheid J. C., Bonting S. L. Studies on (Na+-K+)-activated ATPase. 23. A Mg2+-ATPase in Escherichia coli, activated by monovalent cations. Biochim Biophys Acta. 1969 Mar 18;178(1):128–136. doi: 10.1016/0005-2744(69)90139-9. [DOI] [PubMed] [Google Scholar]
- Hafkenscheid J. C., Bonting S. L. Studies on (Na+-K+)-activated ATPase. XIX. Occurrence and properties of a (Na+-K+)-activated ATPase in Escherichia coli. Biochim Biophys Acta. 1968 Jan 8;151(1):204–211. doi: 10.1016/0005-2744(68)90175-7. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Dio 9 and chlorhexidine: inhibitors of membrane-bound ATPase and of cation transport in Streptococcus faecalis. Biochim Biophys Acta. 1969 Jun 3;183(1):129–136. doi: 10.1016/0005-2736(69)90136-9. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Pavlasova E. Extrusion of sodium and hydrogen ions as the primary process in potassium ion accumulation by Streptococcus faecalis. J Bacteriol. 1970 Jan;101(1):152–159. doi: 10.1128/jb.101.1.152-159.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida M., Mizushima S. Membrane ATPase of Bacillus megaterium. I. Properties of membrane ATPase and its solubilized form. J Biochem. 1969 Jul;66(1):33–43. doi: 10.1093/oxfordjournals.jbchem.a129117. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P. Proton-translocation phosphorylation in mitochondria, chloroplasts and bacteria: natural fuel cells and solar cells. Fed Proc. 1967 Sep;26(5):1370–1379. [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Racker E. Resolution and reconstitution of the inner mitochondrial membrane. Fed Proc. 1967 Sep;26(5):1335–1340. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Thacker W. L., Eagon R. G. Characterization of a membrane-associated ATPase from Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1969 Dec;132(3):1127–1132. doi: 10.3181/00379727-132-34380. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WHEELER K. P. The sensitivity of a kidney ATPase to ouabain and to sodium and potassium. Biochim Biophys Acta. 1961 Aug 19;51:622–624. doi: 10.1016/0006-3002(61)90633-3. [DOI] [PubMed] [Google Scholar]


