Abstract
The pupal defensive secretion of the 24-pointed ladybird beetle, Subcoccinella vigintiquatuorpunctata, consists of a mixture of macrocyclic polyamines, dominated by the three dimeric, 30-membered macrocycles 11-13, derived from the two building blocks 11-(2-hydoxyethylamino)-5-tetradecenoic acid (9) and 11-(2-hydoxyethylamino)-5,8-tetradecadienoic acid (10). Smaller amounts of the four possible cyclic trimers of 9 and 10 were also detected, corresponding to 45-membered macrocycles. Structural assignments were based on NMR-spectroscopic investigations and HPLC–MS analyses. In addition, the all-S absolute configuration of the S. vigintiquatuorpunctata macrocycles was determined by comparison of derivatives of the natural material with enantiomerically pure synthetic samples. Comparing this alkaloid mixture with that of the pupal defensive secretion in related ladybird beetle species indicates that the degree of oligomerization of the 2-hydroxyethylamino carboxylic acid building blocks can be carefully controlled by the insects.
Keywords: alkaloids/chemical defense/Subcoccinella vigintiquatuorpunctata/combinatorial chemistry/pupal secretion
The pupa is a relatively helpless stage in the life cycle of an insect. Unable to crawl or fly, it is potentially vulnerable to any number of predators. In beetles of the family Coccinellidae (ladybird beetles; for recent reviews of the diverse defensive chemistry of coccinellid beetles, see refs. 1 and 2), pupae are endangered further by being conspicuously colored and visually exposed, usually on plant surfaces. We assumed that coccinellid pupae must have means of defense and found this to be the case. In Cycloneda sanguinea (subfamily Coccinellinae), the pupa is armed with so-called “gin traps,” abdominal pinching devices that serve as jaws to fend off ants (ref. 3; Fig. 1 A–C). In pupae of Epilachna (subfamily Epilachninae), the defense is chemical. The pupae in this genus bear a dense coating of tiny glandular hairs, each consisting of a short stalk with a spherical secretory droplet at the tip (Fig. 1 D and E). Ants coming in contact with this fluid are deterred quickly (Fig. 1F).
Figure 1.

(A–C) Pupa of C. sanguinea responding to stimulation with the bristle of a fine paint brush. The jaw-like “gin traps” on the back of the pupa are ordinarily held agape (arrows in A). Insertion of the bristle into a trap causes the pupa to flip upward, with the result that the bristle is “bitten.” (D) Dorsal view of a pupa of Mexican bean beetle (E. varivestis). Note the glandular hairs with glistening droplets of secretion at the tips that fringe the pupa. (E) Enlarged view of glandular hairs of E. varivestis pupa. (F) This ant (Crematogaster cerasi) has just contacted the glandular hairs of an E. borealis pupa (left) with an antenna. It cleans that antenna by brushing it with a foreleg. (Magnification: A–C, ×6; D, ×5; E, ×66; F, ×10).
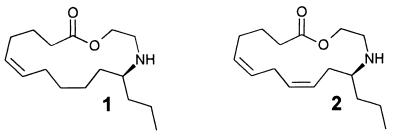
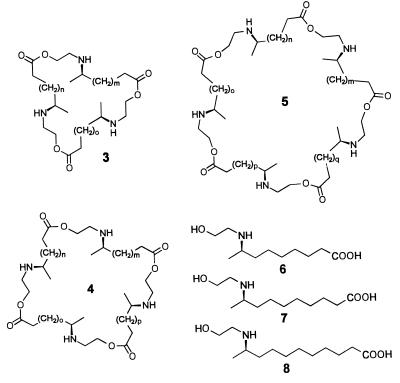
Chemical analysis showed the pupal secretion of one species of Epilachna (E. varivestis, the Mexican bean beetle) to consist of a series of azamacrolides, lactones with a single nitrogen atom incorporated into a ring of 13–17 members. The major components are epilachnene (1) and epilachnadiene (2; Fig. 2; refs. 4 and 5). Studies on the biosynthesis of the azamacrolides showed that epilachnene (1) can be produced from oleic acid and serine (6). The composition of the pupal secretion of another species of Epilachna (E. borealis, the squash beetle), is far more complex, in that it consists of a combinatorial library containing over a hundred macrocyclic polyamines, the polyazamacrolides (PAMLs; for example, 3–5; Fig. 3; refs. 7 and 8). Accompanying the PAMLs are smaller amounts of tocopheryl acetates (9, 10). The PAMLs are derived from an apparently nonselective oligomerization of three (ω − 1)-(2-hydroxyethylamino)alkanoic acids (6–8; Fig. 3), forming macrocycles with well over 200 members (7). Although the biosynthesis of the PAMLs (3–5) has not yet been investigated, the PAMLs clearly seem to be related biosynthetically to the azamacrolides (1, 2) from E. varivestis.
Figure 2.
Epilachnene (1) and epilachnadiene (2) from E. varivestis.
Figure 3.
Principal constituents of the library of PAMLs (3–5) from E. borealis and their building blocks, the (ω − 1)-(2-hydroxyethylamino)alkanoic acids 6–8. In these formulas, each of the variables m–q can have the values 5, 6, or 7.
To see whether nature provides further variants of these defensive materials, we examined the pupal secretion of Subcoccinella vigintiquatuorpunctata (Subcoccinella 24-punctata), another ladybird beetle of the subfamily Epilachninae. We found that, in this species, the pupal secretion consists largely of a mixture of only three dimeric unsaturated PAMLs, based on the same 2-hydroxyethylamino acids as epilachnene (1) and epilachnadiene (2), but built into 30-membered, bis-lactonic rings. Here, we report these findings.‖
EXPERIMENTAL PROCEDURES
Beetles.
Specimens of S. 24-punctata, a European introduction to North American fauna, were collected as adults undergoing reproductive diapause in central Connecticut in September 1997. These beetles were maintained in the laboratory under a natural light cycle until late October. Then, to break diapause, one group was placed outdoors, while another was kept at 4 ± 1°C in constant darkness. In late January 1998, adults from both groups reproduced successfully, when maintained on laboratory-grown Saponaria officinalis, the local host plant (conditions: 22 ± 1°C, 75 ± 5% relative humidity, 14 h light, 10 h darkness). The resulting larvae were also fed S. officinalis grown under identical conditions, yielding pupae for chemical analysis.
Samples.
Our sample of secretion was collected from the glandular hairs of 2- to 4-day-old S. 24-punctata pupae (n = 28) by direct uptake of the droplets with microcapillaries that were rinsed with dichloromethane. The resulting extract was evaporated in vacuo, yielding 100 μg of an oily, colorless residue. A second sample of secretion was obtained by washing whole 2- to 4-day-old S. 24-punctata pupae with 0.2 ml of dichloromethane each. The combined washings of 47 pupae were evaporated in vacuo, yielding 260 μg of an oily, colorless residue.
The samples obtained by these procedures were each dissolved in 0.6 ml of benzene-d6 and subjected directly to NMR analysis. Subsequently, the NMR solvent was removed, and the samples were analyzed by HPLC and GC.
Analytical Procedures.
NMR. Spectra were recorded at 298 K with a Varian Unity+ (500 MHz proton; 126 MHz carbon) spectrometer. For all experiments, benzene-d6 was used as the solvent. Double-quantum filtered correlation spectroscopy and exclusive correlation spectroscopy spectra were acquired by using the standard pulse sequences and phase cycling (11), usually with 512 t1 values, 64 scans per t1 increment, and a sweep width of 5.5 ppm.
HPLC–MS.
A Hewlett–Packard 1090 II pump was linked to a Micromass (Altrincham, U.K.) Quattro I mass spectrometer operated in positive ion-electrospray mode.The HPLC column [250 × 46 mm Inertsil 5 μ ODS-3 (Metachem, Torrance, CA)] was operated at a flow of 1.1 ml/min. The solvent gradient system was from a mixture of 92% water, 5.9% acetonitrile, 2% tetrahydrofuran, and 0.1% formic acid to 66% water, 18.9% acetonitrile, 15% tetrahydrofuran, and 0.1% formic acid over a period of 32 min.
GC–MS.
A Hewlett–Packard HP5890A GC was linked to a Hewlett–Packard mass-selective detector (70 eV electron ionization MS) with a 30-m DB5-MS-coated column (J & W Scientific, Folsom Scientific) with a 0.25-mm i.d. and a 0.25-μm film thickness.
1H NMR Spectroscopic Data of Dimers 11–13.
The chemical shift values of subunit 9 as part of dimer 11 and as part of dimer 12, as well as the chemical shift values of subunit 10 as part of dimer 12 and as part of dimer 13 show only very small differences. Therefore, the three dimers are characterized by way of their building blocks, 9 and 10.
Building block 9 (benzene-d6, 500 MHz): 0.91 (t, J13,14 = 7.4 Hz, 3 H, 14-H), 1.25–1.35 (m, 4H, 12-H and 13-H), 1.63 (m, J2,3 ≈ J3,4 ≈ 7.2, J3,5 = 1.5 Hz, 2 H, 3-H), 2.03 (m, J3,4 ≈ J4,5 ≈ 7.3, J4,6 = 1.5 Hz, 2 H, 4-H), 2.07 (m, J10a,10b = 15, J9,10a = 7.2, J10a,11 = 4.8 Hz, 1 H, 10a-H), 2.16 (m, J9,10b = 7.2, J10b,11 = 5.8 Hz, 1 H, 10b-H), 2.17 (t, J2,3 = 7.2 Hz, 2 H, 2-H), 2.43 (m, 1 H, 11-H), 2.62–2.71 (m, 2 H, CH2-N), 4.14 (ddd, JCHaHbO,CHaHbO = 11, JCHaHbO,CHaHbN = 4.6, JCHaHbO,CHaHbN = 6.9 Hz, 1 H, CHaHbO), 4.21 (ddd, JCHaHbO,CHaHbN = 6.2, JCHaHbO,CHaHbN = 4.5 Hz, 1 H, CHaHbO), 5.30 (m, J5,6 = 10.9 Hz, 1 H, 5-H), 5.38 (m, J8,9 = 10.9 Hz, 1 H, 9-H), 5.44 (m, 1 H, 6-H), 5.49 (m, 1 H, 8-H) ppm.
Building block 10 (benzene-d6, 500 MHz): 0.90 (t, J13,14 = 7.4 Hz, 3 H, 14-H), 1.23–1.36 (m, 10 H, 8-H, 9-H, 10-H, 12-H, 13-H), 1.63 (m, J2,3 ≈ J3,4 ≈ 7.2, J3,5 = 1.5 Hz, 2 H, 3-H), 2.02 (m, J6,7 ≈ J7,8 ≈ 7.3, J5,7 = 1.5 Hz, 2 H, 7-H), 2.03 (m, J3,4 ≈ J4,5 ≈ 7.3, J4,6 = 1.5 Hz, 2 H, 4-H), 2.17 (t, J2,3 = 7.2 Hz, 2 H, 2-H), 2.41 (m, 1 H, 11-H), 2.61–2.72 (m, 2 H, CH2-N), 4.10–4.21 (m, 2 H, CH2O), 5.31 (m, J5,6 = 10.9 Hz, 1 H, 5-H), 5.43 (m, 1 H, 6-H) ppm.
Stereochemical Analysis.
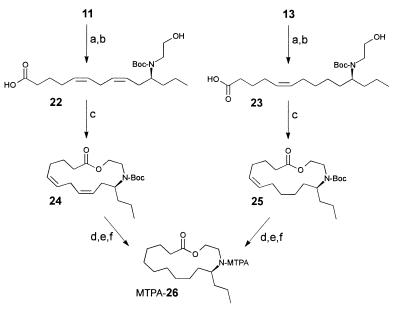
Macrocycles 11 and 13 (isolated by preparative HPLC) were converted into samples of the Mosher derivative of the lactone 26. A solution of macrocycle 11 (about 15 μg) and di-tert-butyl dicarbonate (0.1 mg, 0.5 μmol) in tetrahydrofuran (0.05 ml) was stirred for 3 h at 25°C. After evaporation of the solvent, the bis-tert-butoxycarbonyl-protected macrocycle was hydrolyzed by stirring with a mixture of 2 M aqueous potassium hydroxide solution (0.1 ml) and methanol (0.2 ml) for 6 h at 25°C. After evaporation of most of the methanol and acidification with acetic acid (10 μl), the mixture was extracted with ether (twice with 1 ml). The combined extracts were evaporated. The resulting sample of the hydroxy acid 22 was redissolved in acetonitrile (1 ml), and a large excess of triethylamine (17 mg, 187 μmol) was added. This mixture was then added via syringe pump to a refluxing solution of 2-chloro-1-methylpyridinium iodide (24 mg, 94 μmol) in acetonitrile (5 ml) over a period of 2 h under argon (12). After the addition was complete, the mixture was refluxed for an additional 30 min. The mixture was then evaporated, and the residue was redissolved in a mixture of ether (1 ml) and water (1 ml). The organic layer was separated, filtered through a plug of silica, and evaporated. The resulting sample of N-Boc-protected epilachnadiene (24) was dissolved in 0.1 ml of methanol and then hydrogenated at 1 bar (1 bar = 100 kPa) of H2 by using 50 μg of catalyst (10% palladium on activated carbon). After filtration over a plug of Celite and evaporation, the residue was treated with 10 μl of trifluoroacetic acid in dichloromethane (0.5 ml) for 2 h at 0°C. After the addition of saturated aqueous potassium carbonate solution (0.1 ml), the organic layer was separated and evaporated. Subsequently, the resulting sample of the saturated lactone 26 was derivatized as described (13). The same procedure was used for the conversion of macrocycle 13 into 26.
RESULTS AND DISCUSSION
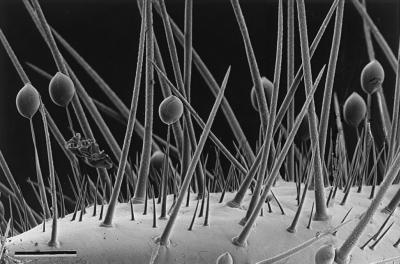
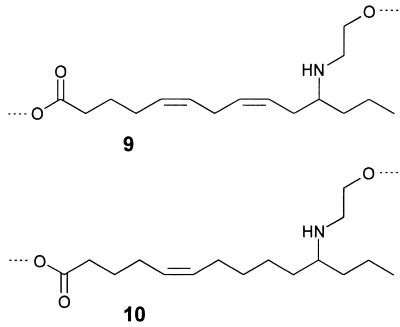
A close-up view of the glandular hairs of S. 24-punctata is shown in Fig. 4. For analyses, we obtained secretion from the hairs by direct uptake of the droplets with microcapillaries and by rinsing whole pupae with organic solvent. To obtain an overview of the secretion’s composition, we started our investigations with direct NMR spectroscopic analyses of the crude, unfractionated natural samples. These analyses indicated that the secretion is composed chiefly of a single group of structurally related compounds, in addition to trace amounts of hydrocarbons and fatty acids. It was shown, by using phase-sensitive (1H,1H) double-quantum filtered correlation spectroscopy and exclusive correlation spectroscopy (11) spectra for the characterization of the proton-spin systems, that the major constituents of the secretion can be derived from two closely related substructures, 9 and 10 (Fig. 5). The 1H chemical shift values and coupling constants of the two methylene groups between the nitrogen and the oxygen indicated that subunits 9 and 10 form cyclic esters, derived from the carbonyl and 2-hydroxyethylamino groups of the two substructures. To corroborate these structural assignments, we carried out an alkaline hydrolysis of the crude secretion, followed by methylation with diazomethane and trifluoroacetylation with trifluoroacetic acid anhydride. This procedure afforded a mixture of the two N,O-bis-trifluoroacetylated methyl esters corresponding to 9 and 10, as was shown by GC–MS comparison of these volatile derivatives with synthetic samples (5).
Figure 4.
Glandular hairs amidst integumental spines on the surface of a S. 24-punctata pupa. (Bar = 100 μm.)
Figure 5.
Substructures 9 and 10 of the major components of the S. 24-punctata pupal secretion.
In the 1H NMR spectra of the secretion, both of the substructures 9 and 10 are represented by two separate sets of signals that show small differences in the chemical shift values and coupling constants of the corresponding protons, suggesting that they both occur as building blocks of more than one component of the secretion. Because the NMR spectroscopic data of the subunits 9 and 10 are different from those of epilachnadiene (2) and epilachnene (1), we reasoned that the components in the S. 24-punctata pupal secretion represent oligomers of 9 and 10 with higher molecular weight. However, the number of these building blocks incorporated into each of the compounds could not be determined on the basis of NMR spectroscopic investigation.
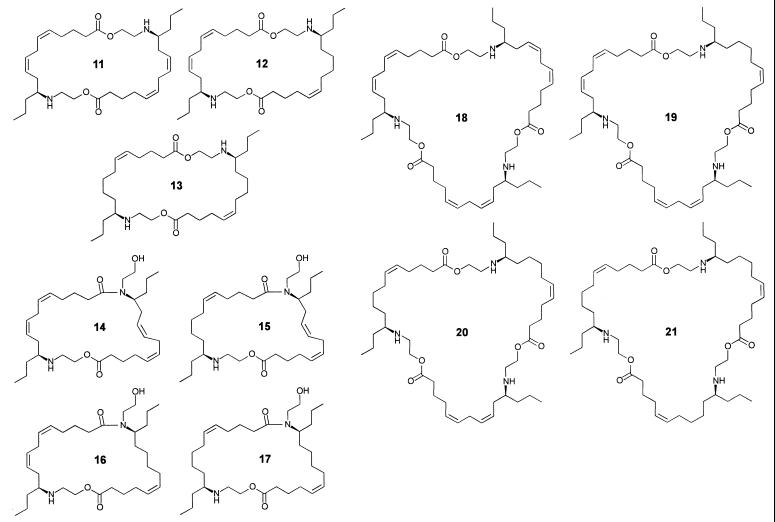
These questions were answered by HPLC–MS analyses, which revealed the secretion to consist mainly of a mixture of the three dimers (11–13) of building blocks 9 and 10 in a ratio of about 4:5:3, respectively (Fig. 6). To corroborate our structural assignment, the three major components 11–13 were isolated by preparative HPLC. 1H NMR spectroscopic analyses of the isolated fractions clearly showed that 11 and 13 represent the symmetric dimers derived from two units of 9 and 10, respectively, whereas component 12 consists of one unit of 9 and one unit of 10 (Fig. 7). Further analysis of the secretion by HPLC–MS showed small amounts of four later eluting isomers (14–17) of the three dimers (Fig. 6). These later eluting isomers showed positive ion-electrospray mass spectra that were strikingly different from those of the major components, 11–13. Whereas under our experimental conditions, the dimers 11–13 show doubly and singly charged pseudomolecular ions, the later eluting isomers 14–17 show almost exclusively singly charged pseudo molecular ions (Fig. 6), suggesting the presence of only one basic nitrogen in each of these compounds. Interestingly, the MS properties of the later eluting dimers in the S. 24-punctata secretion correspond to those of the later eluting isomers of the PAMLs, which we had previously found in the E. borealis pupal secretion and identified as lactams produced by an intramolecular O-to-N acyl transfer in the PAML structures (7). Furthermore, by using the isolated samples of 11–13, we have found that the later eluting isomers, 14–17, form spontaneously. Accordingly, it can be concluded that these later eluting isomers from S. 24-punctata represent the four monoamides that can be derived from intramolecular rearrangement of the dimers 11–13 (Fig. 7).
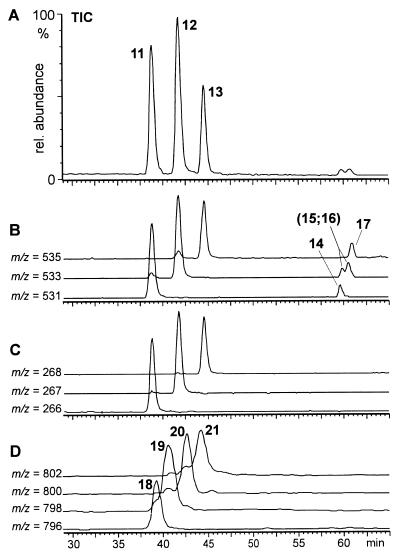
Figure 6.
HPLC-MS-analysis of S. 24-punctata pupal secretion, taken from 2- to 4-day-old pupae. (A) Total ion current (TIC) chromatogram. (B) Ion chromatograms for the pseudo molecular ions (M+H)+ of dimers 11–13 and their isomers 14–17. (C) Ion chromatograms for the doubly charged pseudo molecular ions (M+2H)++ of dimers 11–13. Note that later eluting dimers 14–17 do not show doubly charged ions. (D) Ion chromatograms for the pseudo molecular ions (M+H)+ of trimers 18–21.
Figure 7.
Macrocyclic components identified in the S. 24-punctata defensive secretion.
In addition to the seven dimeric components 11–17, HPLC analysis of the total secretion indicated the presence of trace amounts of the four trimers derivable from 9 and 10 (18–21, Fig. 6). Because these trimers account for less than 2% of the alkaloid mixture, they could not be detected in NMR analyses of the total secretion. Higher oligomers with more than three of the units 9 and 10 as well as the cyclic “monomers” epilachnene (1) and epilachnadiene (2; ref. 4) were not detected in the secretion either by NMR or by GC–MS and HPLC–MS analyses.
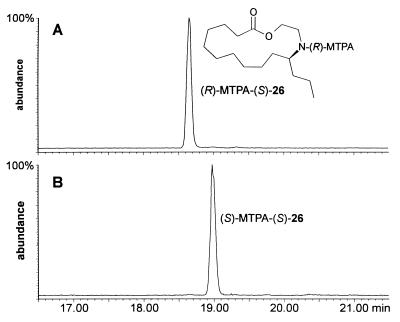
The absolute configuration of macrocycles 11–21 was determined via degradation of the oligomers into derivatives of their building blocks 9 and 10. As shown in Fig. 8, the samples of 11 and 13 that had been isolated by preparative HPLC were used to obtain pure samples of the two hydroxy acids 22 and 23, representing building blocks 9 and 10. The hydroxy acids 22 and 23 were then transformed into derivatives suitable for determination of their absolute configuration by GC. Because of the minute amounts of the natural material available, these synthetic transformations had to be performed on a very small scale. After protecting the nitrogen atoms in 11 and 13 with di-tert-butyl dicarbonate, we subjected the two macrocycles to alkaline hydrolysis, which afforded about 15 μg of each of the hydroxy acids 22 and 23. Because volatile derivatives of the enantiomers of 9 and 10, such as bis-N,O-acetyl-, or trifluoroacetyl derivatives of the corresponding methyl esters, did not separate well on our chiral GC columns, the two hydroxy acids 22 and 23 were separately converted into lactones 24 and 25, which correspond to N-tert-butoxycarbonyl-protected epilachnadiene (2) and epilachnene (1), respectively (Fig. 8). After hydrogenation and deprotection, the two samples of the resulting saturated lactone 26 were converted into the N-α-methoxy-α-trifluoromethylphenylacetyl (MTPA) derivatives (13). As shown in Fig. 9, the diastereomeric MTPA amides of 26 are well separated by GC. GC comparison of the MTPA amides of 26 derived from the macrocycles 11 and 13 with MTPA amides of 26 synthesized from enantiomerically pure (S)-epilachnene (1; ref. 5) indicated that building blocks 9 and 10 both have the (S)-configuration with at least 98% enantiomeric excess. Thus, the absolute configuration of building blocks 9 and 10 of the S. 24-punctata oligomers is identical to that of the corresponding monomeric lactone epilachnene (1) from E. varivestis (5).
Figure 8.
Synthesis of lactone 26 from isolated samples of dimers 11 and 13. Step a, (Boc)2O, tetrahydrofuran 3 h at 25°C. Step b, 2 M aqueous KOH, CH3OH, 6 h at 25°C. Step c, 2-chloro-1-methylpyridinium iodide, triethylamine, CH3CN, 80°C, high dilution (12). Step d, 10% Pd/C, H2, CH3OH. Step e, CF3COOH, CH2Cl2, 2 h at 0°C. Step f, see ref. 13.
Figure 9.
Assignment of absolute configuration of lactone 26 by GC comparison of the corresponding Mosher derivative. (A) (R)-MTPA derivative of (S)-26 derived from synthetic (S)-epilachnene (1). (B) (S)-MTPA derivative of 26 derived from natural 11. The GC column was J & W Scientific 29 m fused silica DB5-MS with a film thickness of 0.25 μm and i.d. of 0.25 mm; temperatures started at 150°C then increased at a rate of 10°C/min to 290°C.
The S. 24-punctata pupal secretion consists largely of a set of unsaturated PAMLs derived from building blocks 9 and 10, dominated by dimeric alkaloids 11–13. As in the case of the saturated PAMLs from E. borealis, the quantitative distribution of PAMLs in S. 24-punctata suggests that building blocks 9 and 10 are incorporated into these oligomers in random fashion. These two secretions differ significantly, however, in that E. borealis produces a complex series of oligomers, in which even a 20-mer (280-membered ring) can be detected (7), whereas S. 24-punctata produces chiefly dimers accompanied by only trace amounts of trimers. Comparing the pupal secretion of S. 24-punctata with that of E. varivestis, it seems that, although both species use identical building blocks (9 and 10), the alkaloid mixtures produced by each species have no overlapping constituents. The two cyclic monomers epilachnene (1) and epilachnadiene (2) are absent from the alkaloid mixture of S. 24-punctata, and not even traces of the dimers 11–13, the corresponding lactams 14–17, or the trimers 18–21 are present in the E. varivestis secretion (4). We specifically reexamined a sample of E. varivestis secretion to establish this point. Thus, it is apparent that the degree of oligomerization of the building blocks in these pupal defensive secretions is controlled carefully and conceivably could represent a specific adaptation optimizing the deterrence of the fluid against particular predators.
Acknowledgments
We thank V. Salvador for assistance and T. Begley for comments on the manuscript. This work was supported in part by National Institutes of Health Grants GM53830 and AI02908 and by Deutsche Forschungsgemeinschaft Grant Schr609/1-1.
ABBREVIATIONS
- MTPA
N-α-methoxy-α-trifluoromethylphenylacetyl
- PAML
polyazamacrolide
- Subcoccinella 24-punctata
Subcoccinella vigintiquatuorpunctata
Footnotes
This paper is no. 155 in the series “Defense Mechanisms of Arthropods”; paper no. 154 is ref. 14.
References
- 1.Daloze D, Braekman J-C, Pasteels J M. Chemoecology. 1995;5/6:173–183. [Google Scholar]
- 2.King A G, Meinwald J. Chem Rev (Washington, DC) 1996;96:1105–1122. doi: 10.1021/cr950242v. [DOI] [PubMed] [Google Scholar]
- 3.Eisner T, Eisner M. Psyche. 1992;99:265–274. [Google Scholar]
- 4.Attygalle A B, McCormick K D, Blankespoor C L, Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1993;90:5204–5208. doi: 10.1073/pnas.90.11.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer J J, Attygalle A B, Smedley S R, Eisner T, Meinwald J. Tetrahedron Lett. 1997;38:2787–2790. [Google Scholar]
- 6.Attygalle A B, Blankespoor C L, Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1994;91:12790–12793. doi: 10.1073/pnas.91.26.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schröder F C, Farmer J J, Attygalle A B, Smedley S R, Eisner T, Meinwald J. Science. 1998;281:428–431. doi: 10.1126/science.281.5375.428. [DOI] [PubMed] [Google Scholar]
- 8.Schröder F C, Farmer J J, Smedley S R, Eisner T, Meinwald J. Tetrahedron Lett. 1998;39:6625–6628. [Google Scholar]
- 9.Attygalle A B, Smedley S R, Eisner T, Meinwald J. Experientia. 1996;52:616–620. doi: 10.1007/BF01969741. [DOI] [PubMed] [Google Scholar]
- 10.Attygalle A B, Meinwald J, Rossini C, Eisner T. Naturwissenschaften. 1996;83:277–279. doi: 10.1007/BF01149602. [DOI] [PubMed] [Google Scholar]
- 11.Griesinger C, Sørensen O W, Ernst R R. J Magn Reson. 1987;75:474–492. [Google Scholar]
- 12.Mukaiyama, T., Narasaka, K. & Kikuchi, K. (1977) Chem. Lett. 441–444.
- 13.Dale J A, Dull D L, Mosher H S. J Org Chem. 1969;34:2543–2549. [Google Scholar]
- 14.Yang Z-C, Meinwald J. Tetrahedron Lett. 1998;39:3425–3428. [Google Scholar]