Abstract
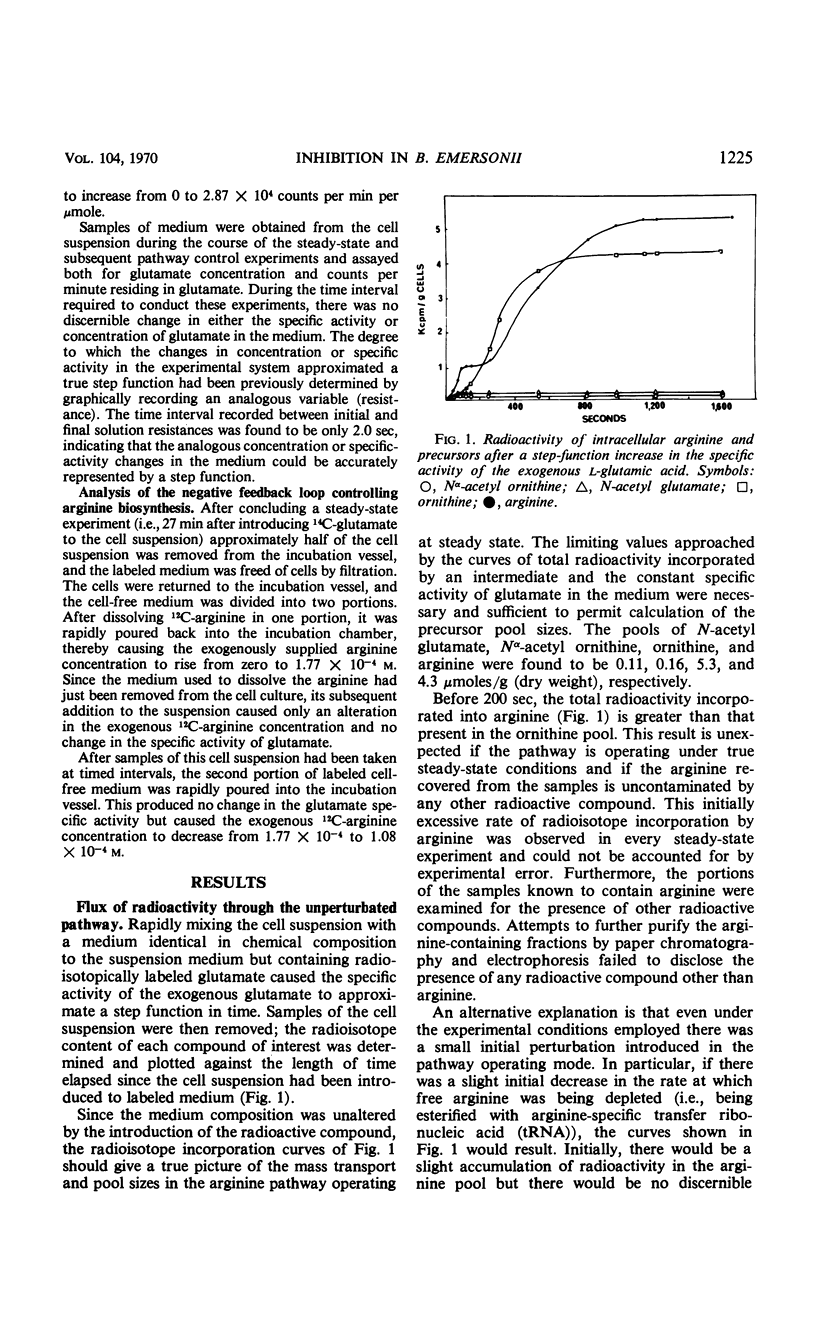
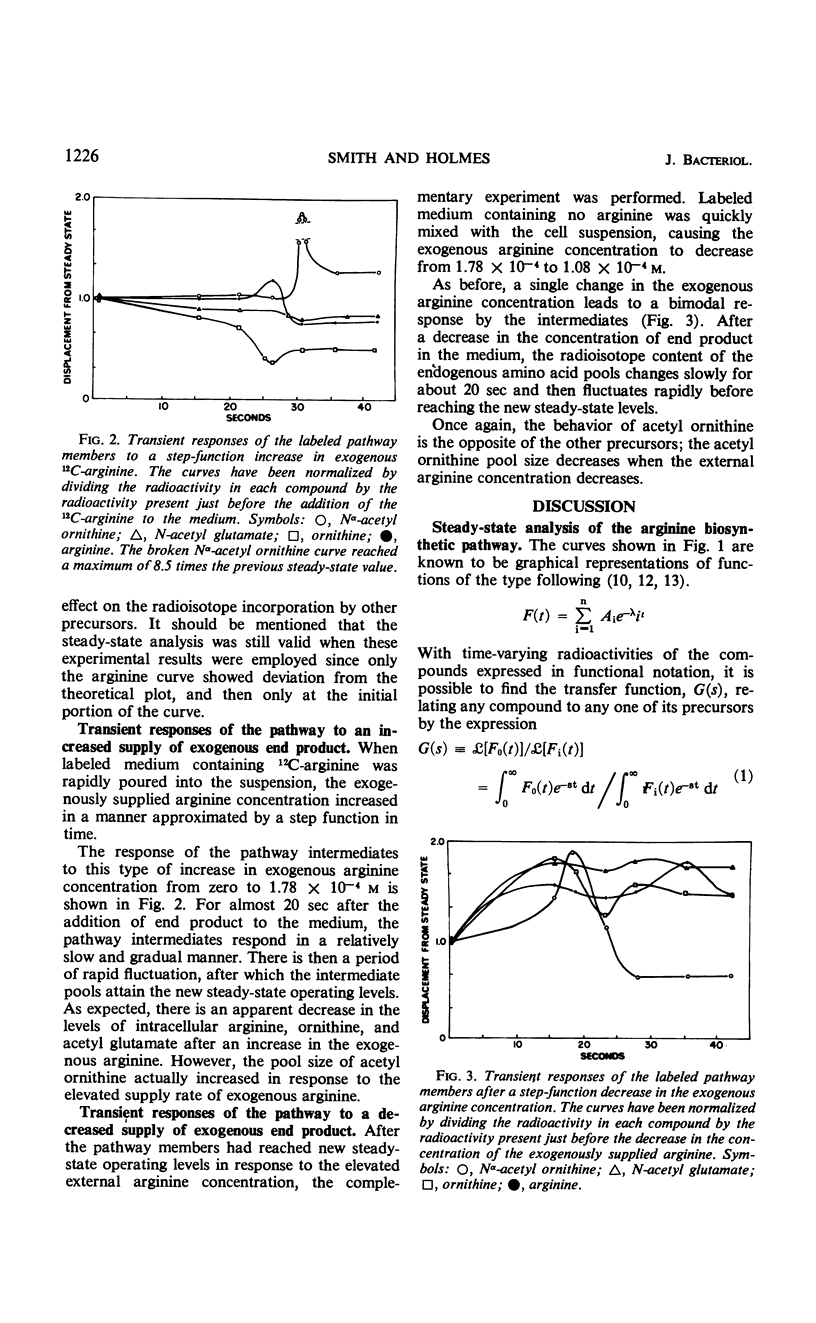
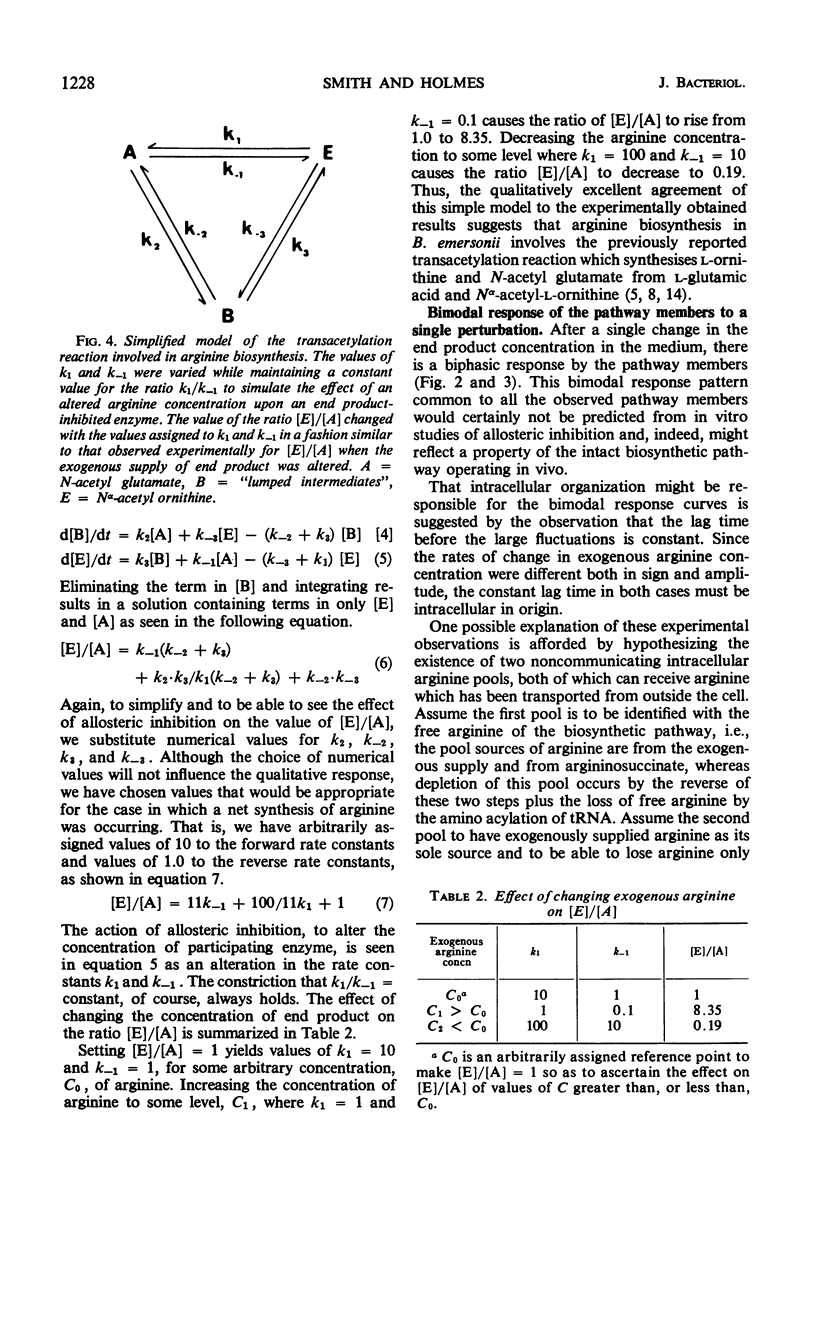
Negative feedback control of the arginine biosynthetic pathway of Blastocladiella emersonii was observed in vivo by conducting kinetic radioisotope incorporation experiments. Transient responses of arginine precursor pools to perturbations in the medium end product concentration allowed a quantitative description of the negative feedback associated with pathway control, indicated that the Nα-acetyl-l-glutamate pool size was the controlled variable, and suggested that Nα-acetyl-l-glutamate and l-ornithine were the products of a transacetylation reaction in which Nα-acetyl-l-ornithine and glutamic acid were the reactants. The precursors to arginine biosynthesis were found to undergo two discrete disturbances in response to altering the exogenous arginine concentration only once. An increased supply of exogenous end product resulted in diminished radioactivity in the intracellular pools of arginine, ornithine, and acetyl-glutamate, but elevated the radioisotope content of the pool of Nα-acetyl-l-ornithine. Conversely, a lowered concentration of end product in the medium caused a rise in the radioisotope content of all the precursor pools except that of Nα-acetyl ornithine, in which the radioactivity fell. Examination of tracer distribution among the precursors in the absence of exogenous arginine showed the nonacetylated precursor pools to be about 30 times larger than those of the acetylated intermediates and placed upper limits on the pool sizes of two pathway members that were not isolated (Nα-acetyl-l-glutamic-γ-semialdehyde and Nα-acetyl-l-glutamic-γ-semiphosphate).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- MIDDELHOVEN W. J. THE ORNITHINE PATHWAY IN THE YEAST CANDIDA UTILIS. Biochim Biophys Acta. 1963 Sep 3;77:152–154. doi: 10.1016/0006-3002(63)90482-7. [DOI] [PubMed] [Google Scholar]
- MYHILL J., WADSWORTH G. P., BROWNELL G. L. INVESTIGATION OF AN OPERATOR METHOD IN THE ANALYSIS OF BIOLOGICAL TRACER DATA. Biophys J. 1965 Jan;5:89–107. doi: 10.1016/s0006-3495(65)86704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RESCIGNO A. ON SOME TOPOLOGICAL PROPERTIES OF THE SYSTEMS OF COMPARTMENTS. Bull Math Biophys. 1964 Mar;26:31–38. doi: 10.1007/BF02476618. [DOI] [PubMed] [Google Scholar]
- Vogel H. J. Path of Ornithine Synthesis in Escherichia Coli. Proc Natl Acad Sci U S A. 1953 Jul;39(7):578–583. doi: 10.1073/pnas.39.7.578. [DOI] [PMC free article] [PubMed] [Google Scholar]



