Abstract
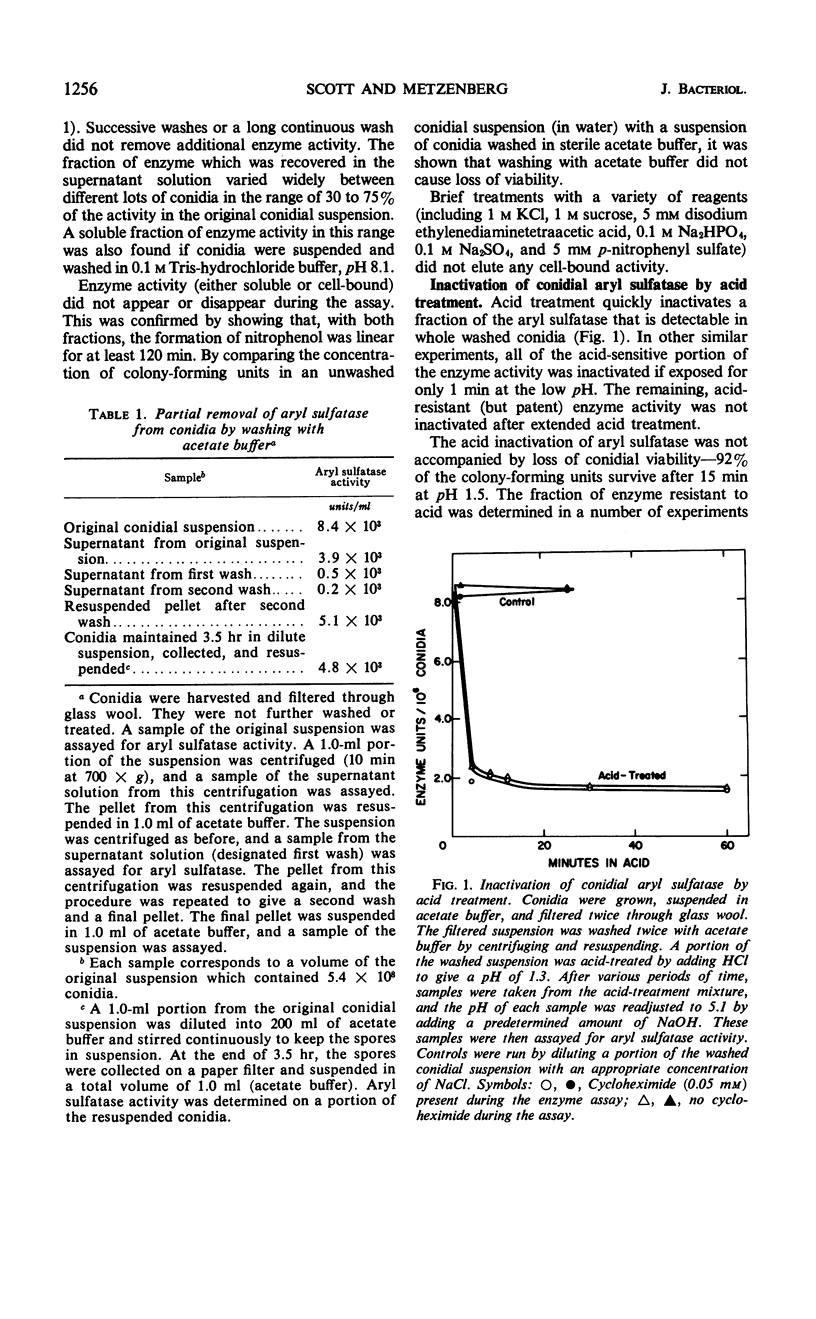
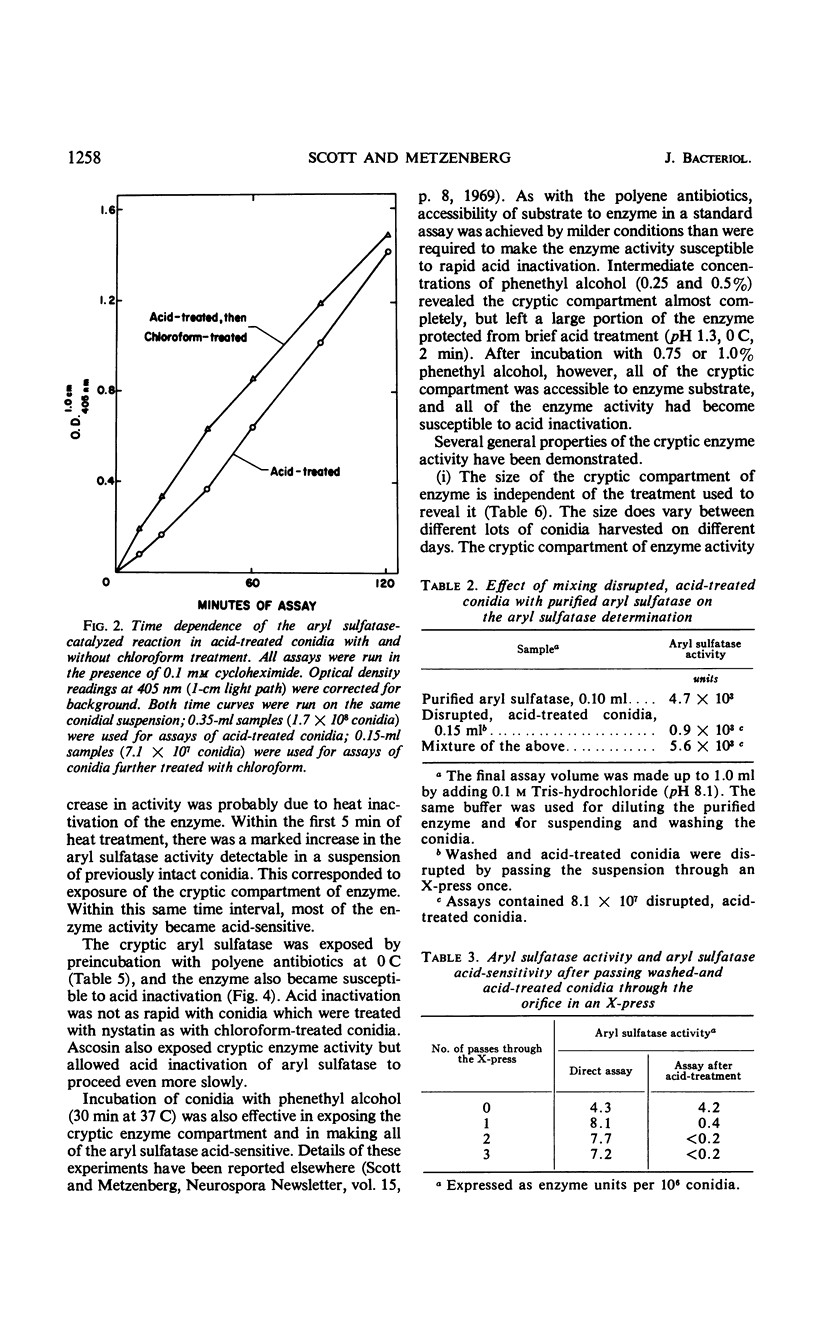
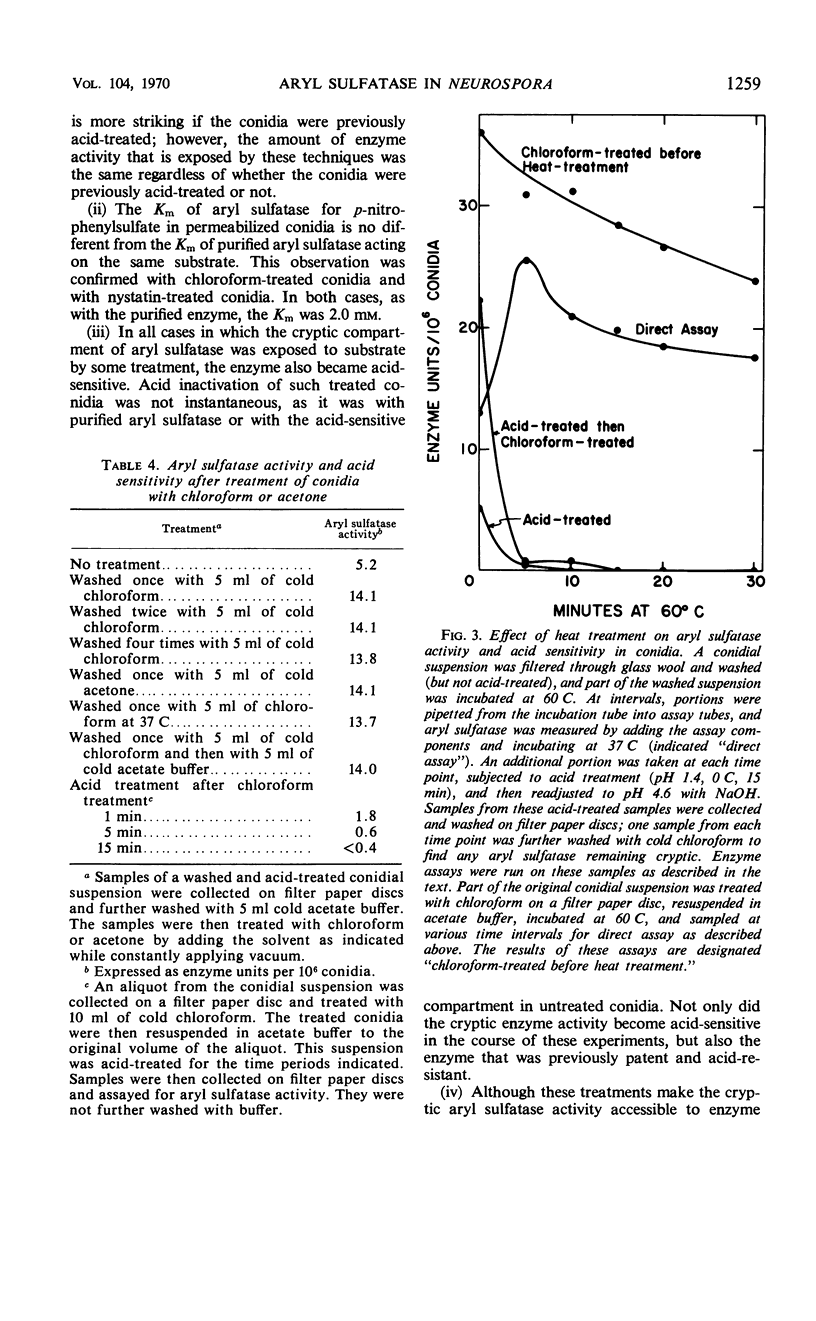
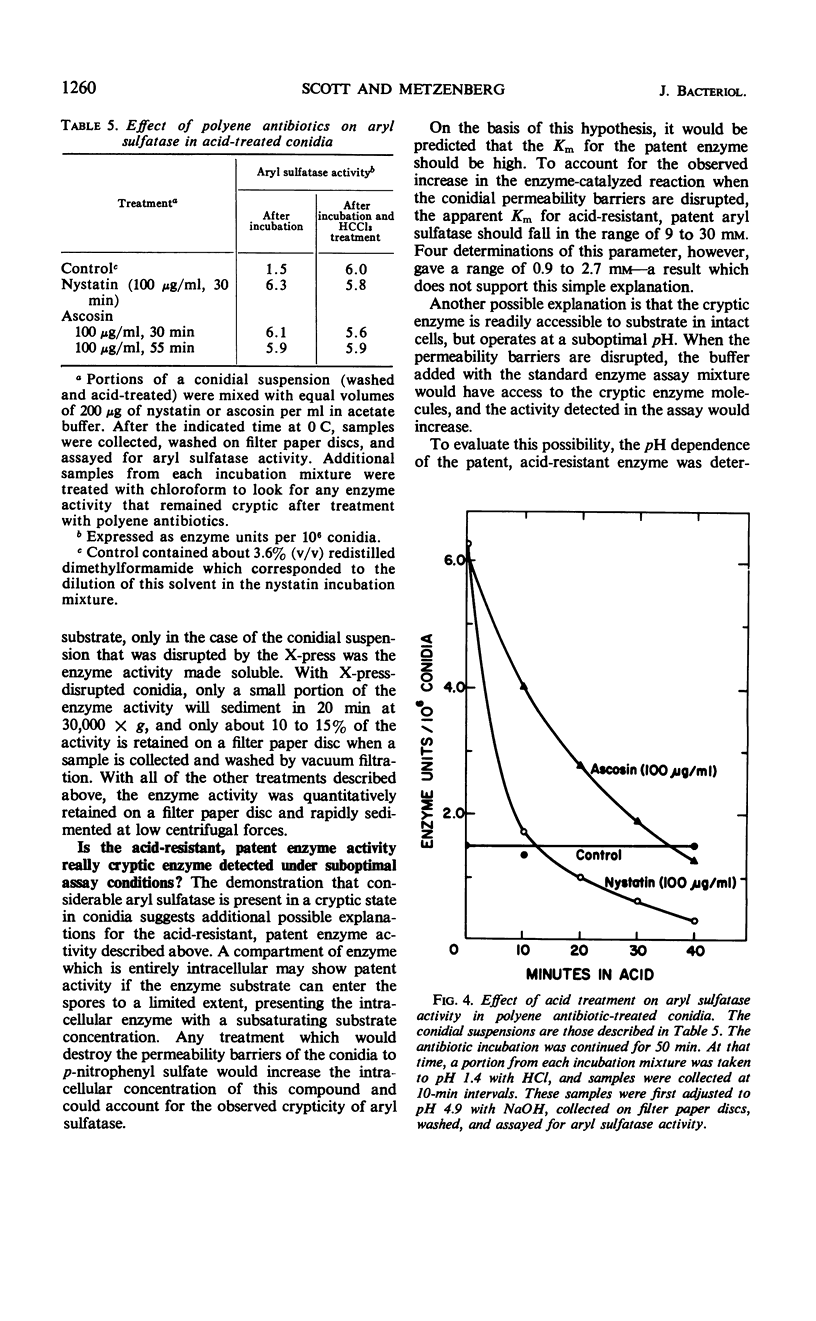
Aryl sulfatase (arylsulfate sulfohydrolase, EC 3.1.6.1) was found to have multiple locations in Neurospora conidia. Some enzyme activity remained in the supernatant when a spore suspension was centrifuged or filtered. Part of the cell-bound activity could be detected by adding the assay ingredients to a suspension of intact spores (patent enzyme), and additional activity was only detectable when the spores were first treated to destroy their permeability barriers (cryptic enzyme). Such treatments include: disruption with an X-press, brief rinsing with chloroform or acetone, incubation at 60 C for 5 min, and incubation with phenethyl alcohol, nystatin, or ascosin. Part of the patent aryl sulfatase was inactivated by briefly acid treating the intact spores (no loss of conidial viability). This enzyme was considered to have a cell surface location. Some enzyme was acid-resistant in intact spores, but all of the enzyme was acid-sensitive in spores whose permeability barriers had been disrupted. The pH dependence, kinetic properties, and p-nitrophenyl sulfate uptake were investigated in acid-treated conidia. No aryl sulfatase was detected in ascospores. Young mycelia contained more aryl sulfatase than did conidia, but little, if any, was secreted into the growth medium. Cryptic activity was demonstrated in young mycelia by brief chloroform treatment or by rinsing the cells with 0.1 m acetate buffer. Enzyme activity in young mycelia was completely labile to acid treatment, as was cell viability.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EBERHART B. M. Exogenous enzymes of Neurospora conidia and mycelia. J Cell Comp Physiol. 1961 Aug;58:11–16. doi: 10.1002/jcp.1030580103. [DOI] [PubMed] [Google Scholar]
- Eberhart B. M., Beck R. S. Localization of the beta-glucosidases in Neurospora crassa. J Bacteriol. 1970 Feb;101(2):408–417. doi: 10.1128/jb.101.2.408-417.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascón S., Lampen J. O. Purification of the internal invertase of yeast. J Biol Chem. 1968 Apr 10;243(7):1567–1572. [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Günther T., Kattner W., Merker H. J. Uber das Verhalten und die Lokalisation der suaren Phosphatase von Hefezellen bei Repression und Derepression. Exp Cell Res. 1967 Jan;45(1):133–147. doi: 10.1016/0014-4827(67)90118-8. [DOI] [PubMed] [Google Scholar]
- HEREDIA C. F., YEN F., SOLS A. Role and formation of the acid phosphatase in yeast. Biochem Biophys Res Commun. 1963 Jan 18;10:14–18. doi: 10.1016/0006-291x(63)90259-6. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Horikoshi K., Ikeda Y. Studies on the spore coats of aspergillus oryzae. II. Conidia coat-bound beta-glucosidase. Biochim Biophys Acta. 1965 Nov 1;101(3):352–357. doi: 10.1016/0926-6534(65)90014-x. [DOI] [PubMed] [Google Scholar]
- Johnson H. N., DeBusk A. G. The beta-galactosidase system of Neurospora crassa. II. Extracellular nature of the pH 4.2 enzyme. Arch Biochem Biophys. 1970 Jun;138(2):412–417. doi: 10.1016/0003-9861(70)90364-4. [DOI] [PubMed] [Google Scholar]
- KINSKY S. C. Nystatin binding by protoplasts and a particulate fraction of Neurospora crassa, and a basis for the selective toxicity of polyene antifungal antibiotics. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1049–1056. doi: 10.1073/pnas.48.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappy M. S., Metzenberg R. L. Multiple alterations in metabolite uptake in a mutant of Neurospora crassa. J Bacteriol. 1967 Nov;94(5):1629–1637. doi: 10.1128/jb.94.5.1629-1637.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester G. Inhibition of Growth, Synthesis, and Permeability in Neurospora crassa by Phenethyl Alcohol. J Bacteriol. 1965 Jul;90(1):29–37. doi: 10.1128/jb.90.1.29-37.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELS G. R. Localization of carbohydrases at the surface of fungus spores by acid treatment. Exp Cell Res. 1953 Sep;5(1):48–55. doi: 10.1016/0014-4827(53)90093-7. [DOI] [PubMed] [Google Scholar]
- MATILE P. INTRAZELLULAERE LOKALISATION PROTEOLYTISCHER ENZYME VON NEUROSPORA CRASSA. I. FUNKTION UND SUBZELLULAERE VERTEILUNG PROTEOLYTISCHER ENZYME. Z Zellforsch Mikrosk Anat. 1965 Mar 16;65:884–896. [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- Maddox I. S., Hough J. S. Proteolytic enzymes of Saccharomyces carlsbergensis. Biochem J. 1970 May;117(5):843–852. doi: 10.1042/bj1170843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan P. R., Menon C. P. Laminarinase of Neurospora crassa. I. Enzyme activity associated with conidia & conidial wall. Indian J Biochem. 1968 Mar;5(1):6–8. [PubMed] [Google Scholar]
- Marzluf G. A., Metzenberg R. L. Positive control by the cys-3 locus in regulation of sulfur metabolism in Neurospora. J Mol Biol. 1968 Apr 28;33(2):423–437. doi: 10.1016/0022-2836(68)90199-x. [DOI] [PubMed] [Google Scholar]
- Matile P. Prospects of yeast cytology. Antonie Van Leeuwenhoek. 1969 Jun;35(Suppl):59–70. [PubMed] [Google Scholar]
- Metzenberg R. L., Ahlgren S. K. Mutants of Neurospora deficient in aryl sulfatase. Genetics. 1970 Mar-Apr;64(3):409–422. [PMC free article] [PubMed] [Google Scholar]
- Metzenberg R. L., Ahlgren S. K. Preparation of S35-labeled p-nitrophenyl sulfate and its use in assay of low levels of aryl sulfatase. Eur J Cancer. 1970 Apr;6(2):523–526. doi: 10.1016/0003-2697(70)90216-2. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L., Parson J. W. Altered repression of some enzymes of sulfur utilization in a temperature-conditional lethal mutant of Neurospora. Proc Natl Acad Sci U S A. 1966 Mar;55(3):629–635. doi: 10.1073/pnas.55.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzenberg R. L. Repair of multiple defects of a regulatory mutant of Neurospora by high osmotic pressure and by reversion. Arch Biochem Biophys. 1968 May;125(2):532–541. doi: 10.1016/0003-9861(68)90611-5. [DOI] [PubMed] [Google Scholar]
- Neal A. L., Weinstock J. O., Lampen J. O. Mechanisms of Fatty Acid Toxicity for Yeast. J Bacteriol. 1965 Jul;90(1):126–131. doi: 10.1128/jb.90.1.126-131.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyc J. F. A repressible acid phosphatase in Neurospora crassa. Biochem Biophys Res Commun. 1967 Apr 20;27(2):183–188. doi: 10.1016/s0006-291x(67)80059-7. [DOI] [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Wendt L. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. J Bacteriol. 1967 Feb;93(2):560–566. doi: 10.1128/jb.93.2.560-566.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai N., Uchida T., Egami F. Purification and properties of ribonuclease N1, an extracellular ribonuclease of Neurospora crassa. Biochim Biophys Acta. 1966 Oct 17;128(1):218–220. doi: 10.1016/0926-6593(66)90168-8. [DOI] [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. EVIDENCE FOR AN EXOCELLULAR SITE FOR THE ACID PHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1964 Dec;88:1743–1754. doi: 10.1128/jb.88.6.1743-1754.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALOKAR M. Cytochemistry of centrifuged hyphae of Neurospora. Exp Cell Res. 1960 Feb;19:114–132. doi: 10.1016/0014-4827(60)90042-2. [DOI] [PubMed] [Google Scholar]


