Abstract
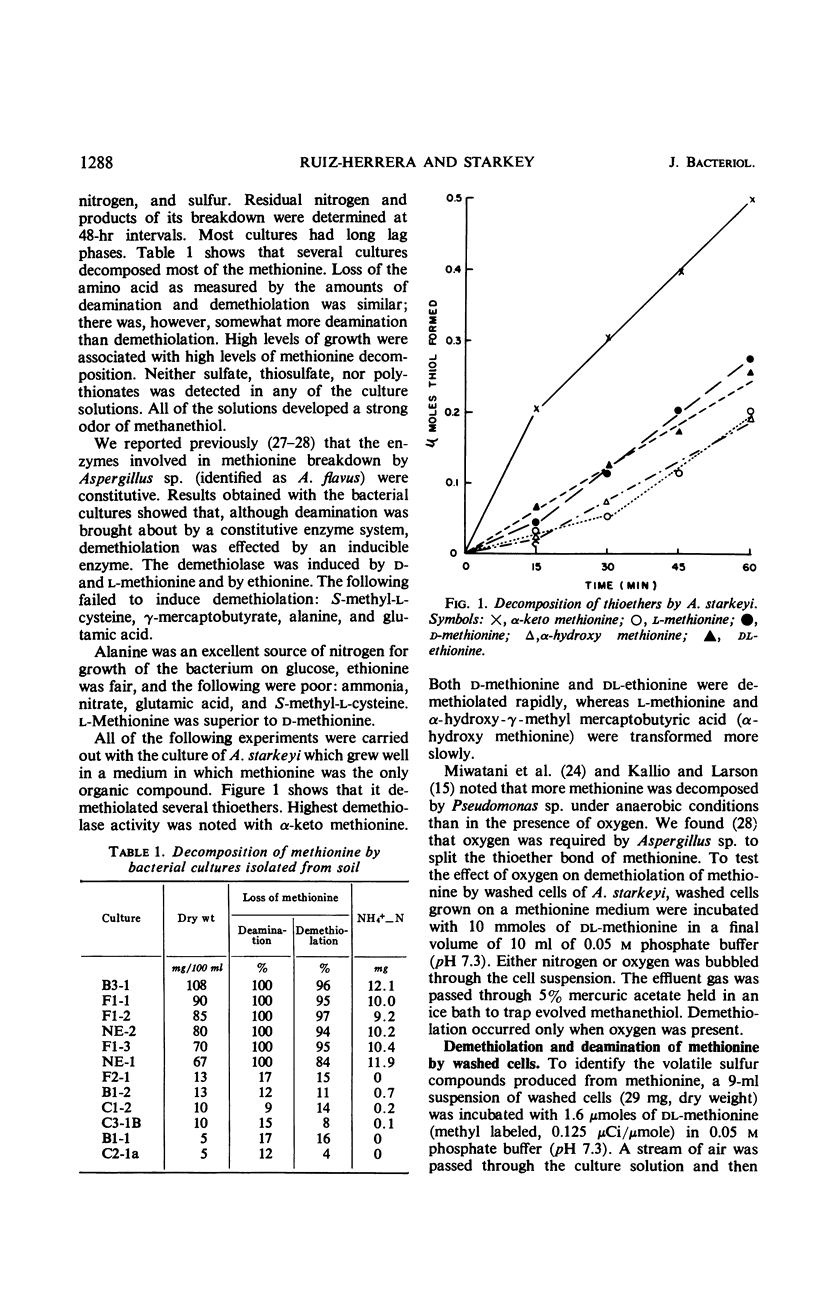
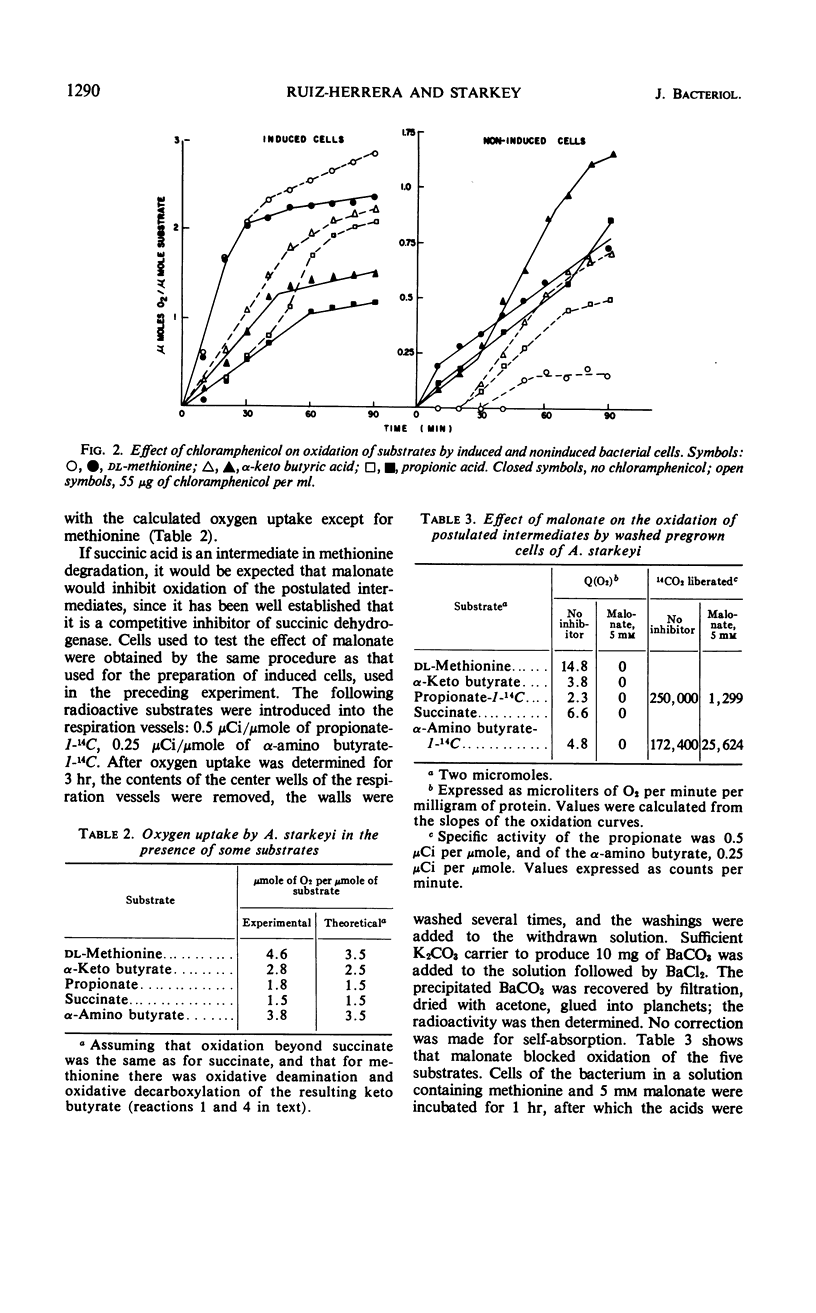
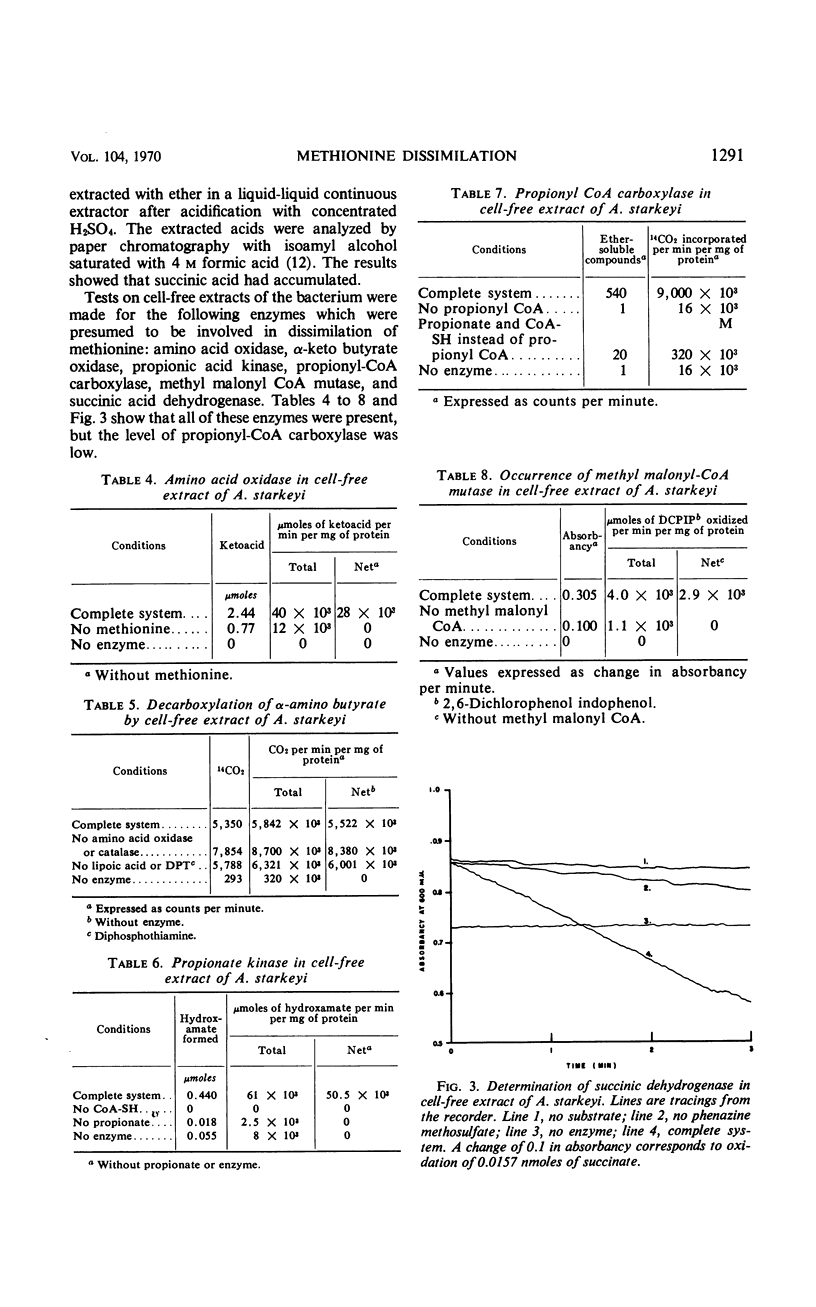
Methionine was decomposed by some bacteria which were isolated from soil. The sulfur of the methionine was liberated as methanethiol, and part of this became oxidized to dimethyl disulfide. Detailed studies with one of these cultures, Achromobacter starkeyi, indicated that the first step in methionine decomposition was its oxidadative deamination to α-keto-γ-methyl mercaptobutyrate by a constitutive amino acid oxidase. The following steps were carried out by inducible enzymes, the synthesis of which was inhibited by chloramphenicol. α-Keto-γ-methyl mercaptobutyrate was split producing methanethiol and α-keto butyrate which was oxidized to propionate. The metabolism of propionate was similar to that described for animal tissues; the propionate was carboxylated to succinate via methyl malonyl coenzyme A, and the succinate was metabolized through the Krebs cycle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK W. S., FLAVIN M., OCHOA S. Metabolism of propionic acid in animal tissues. III. Formation of succinate. J Biol Chem. 1957 Dec;229(2):997–1010. [PubMed] [Google Scholar]
- BECK W. S., OCHOA S. Metabolism of propionic acid in animal tissues. IV. Further studies on the enzymatic isomerization of methylmalonyl coenzyme A. J Biol Chem. 1958 Jun;232(2):931–938. [PubMed] [Google Scholar]
- CANELLAKIS E. S., TARVER H. Studies on protein synthesis in vitro. IV. Concerning the apparent uptake of methionine by particulate preparation from liver. Arch Biochem Biophys. 1953 Feb;42(2):387–398. doi: 10.1016/0003-9861(53)90367-1. [DOI] [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- FLAVIN M., CASTRO-MENDOZA H., OCHOA S. Metabolism of propionic acid in animal tissues. II. Propionyl coenzyme a carboxylation system. J Biol Chem. 1957 Dec;229(2):981–996. [PubMed] [Google Scholar]
- FLAVIN M., OCHOA S. Metabolism of propionic acid in animal tissues. I. Enzymatic conversion of propionate to succinate. J Biol Chem. 1957 Dec;229(2):965–979. [PubMed] [Google Scholar]
- FLAVIN M., ORTIZ P. J., OCHOA S. Metabolism of propionic acid in animal tissues. Nature. 1955 Oct 29;176(4487):823–826. doi: 10.1038/176823a0. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., ADLER J. Synthesis of succinate from propionate and bicarbonate by soluble enzymes from liver mitochondria. J Biol Chem. 1956 Apr;219(2):933–942. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MITSUHASHI S. Decomposition of thioether derivatives by bacteria; methylmercaptan formation and the properties of the responsible enzyme. Jpn J Exp Med. 1949 Sep;20(2):211–222. [PubMed] [Google Scholar]
- Ruiz-Herrera J., Starkey R. L. Dissimilation of methionine by a demethiolase of Aspergillus species. J Bacteriol. 1969 Sep;99(3):764–770. doi: 10.1128/jb.99.3.764-770.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Starkey R. L. Dissimilation of methionine by fungi. J Bacteriol. 1969 Aug;99(2):544–551. doi: 10.1128/jb.99.2.544-551.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal W., Starkey R. L. Microbial decomposition of methionine and identity of the resulting sulfur products. J Bacteriol. 1969 Jun;98(3):908–913. doi: 10.1128/jb.98.3.908-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESENDANGER S., NISMAN B. La Lméthionine démercapto-désaminase: un nouvel enzyme à pyridoxal-phosphate. C R Hebd Seances Acad Sci. 1953 Oct 5;237(14):764–765. [PubMed] [Google Scholar]



