Abstract
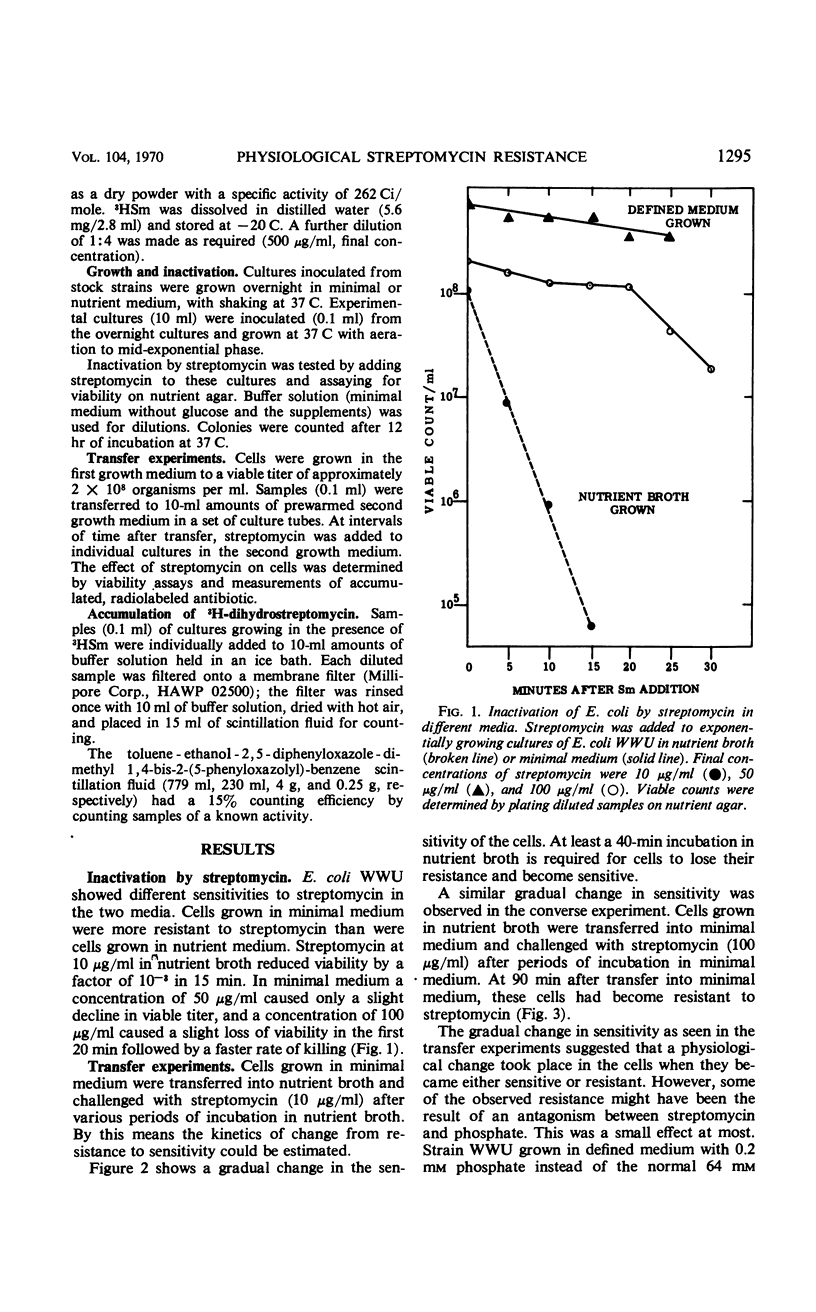
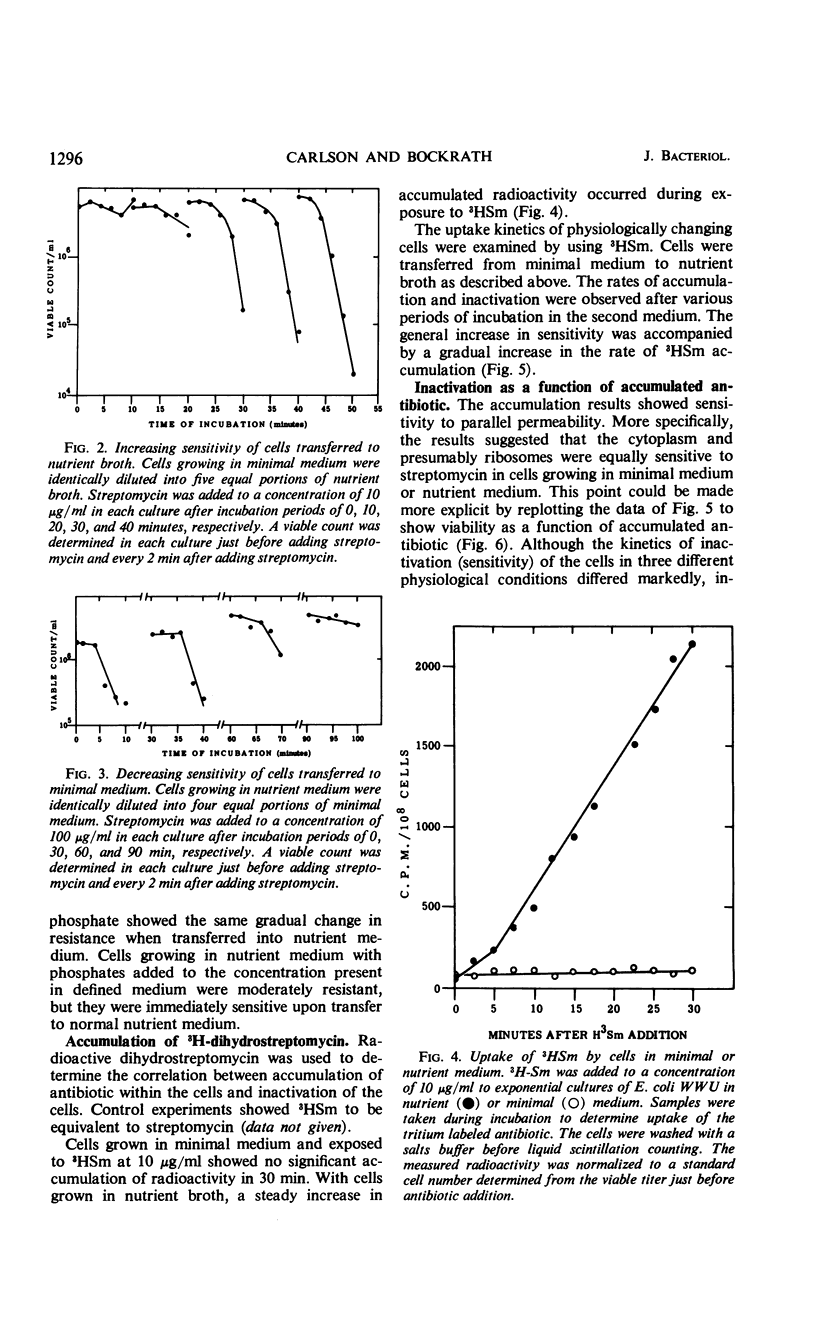
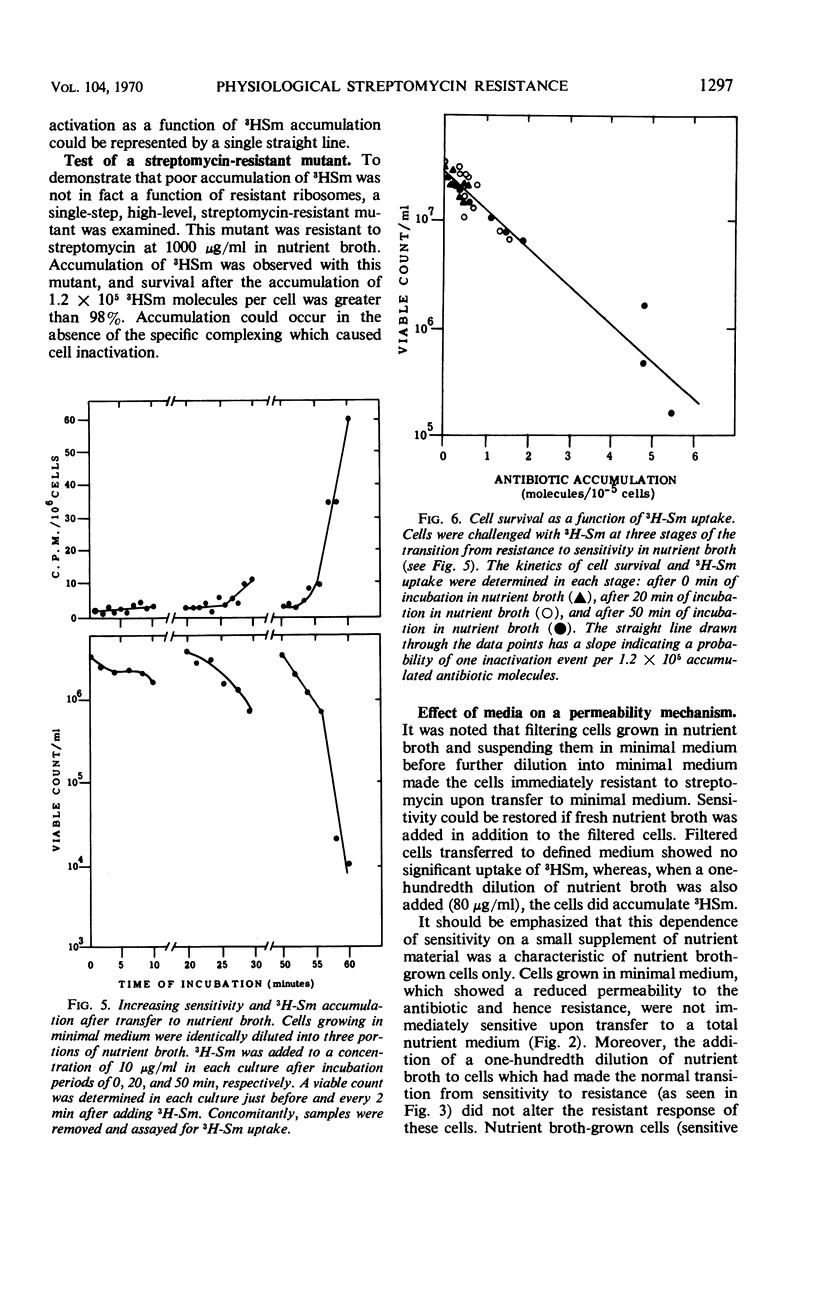
Escherichia coli strain WWU was found to be moderately resistant to streptomycin when grown in a minimal medium, although the strain was sensitive if grown in nutrient broth. Transfer experiments showed that cells grown in minimal medium retain the resistant state for a period of time after dilution into nutrient broth; and conversely, sensitive cells grown in nutrient broth were sensitive after dilution into minimal medium for a period of time. The kinetics of transition from resistant to sensitive and from sensitive to resistant were observed, and kinetics of 3H-dihydrostreptomycin accumulation by resistant and sensitive cells were compared. The data suggested that cells grown in minimal medium were physiologically resistant because they accumulated streptomycin poorly. Inactivation per incorporated antibiotic molecule was the same in resistant and sensitive cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockrath R. C., Osborn M., Person S. Nonsense suppression in a multiauxotrophic derivative of Escherichia coli 15T-: identification and consequences of an amber triplet in the deoxyribomutase gene. J Bacteriol. 1968 Jul;96(1):146–153. doi: 10.1128/jb.96.1.146-153.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen W. B. Reduced streptomycin killing in E. coli carrying the Mu-factor in its extrachromosomal state. Acta Pathol Microbiol Scand. 1965;65(4):627–635. doi: 10.1111/apm.1965.65.4.627. [DOI] [PubMed] [Google Scholar]
- Harwood J. H., Smith D. H. Resistance factor-mediated streptomycin resistance. J Bacteriol. 1969 Mar;97(3):1262–1271. doi: 10.1128/jb.97.3.1262-1271.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Streptomycin Resistance in Escherichia Coli Analyzed by Transduction. Genetics. 1960 Jan;45(1):49–62. doi: 10.1093/genetics/45.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- PLOTZ P. H., DUBIN D. T., DAVIS B. D. Influence of salts on the uptake of streptomycin by Escherichia coli. Nature. 1961 Sep 23;191:1324–1325. doi: 10.1038/1911324a0. [DOI] [PubMed] [Google Scholar]
- SPOTTS C. R., STANIER R. Y. Mechanism of streptomycin action on bacteria: a unitary hypothesis. Nature. 1961 Nov 18;192:633–637. doi: 10.1038/192633a0. [DOI] [PubMed] [Google Scholar]
- TZAGOLOFF H., UMBREIT W. W. Influence of streptomycin on nucleotide excretion in Escherichia coli. J Bacteriol. 1963 Jan;85:49–52. doi: 10.1128/jb.85.1.49-52.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasawa S., Utahara R., Okanishi M., Maeda K., Umezawa H. Studies on adenylylstreptomycin, a product of streptomycin inactivation by E. coli carrying the R-factor. J Antibiot (Tokyo) 1968 Aug;21(8):477–484. doi: 10.7164/antibiotics.21.477. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Streptomycin resistance mutation in Escherichia coli: altered ribosomal protein. Science. 1968 Apr 12;160(3824):198–199. doi: 10.1126/science.160.3824.198. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Takasawa S., Okanishi M., Utahara R. Adenylylstreptomycin, a product of streptomycin inactivated by E. coli carrying R factor. J Antibiot (Tokyo) 1968 Jan;21(1):81–82. doi: 10.7164/antibiotics.21.81. [DOI] [PubMed] [Google Scholar]


