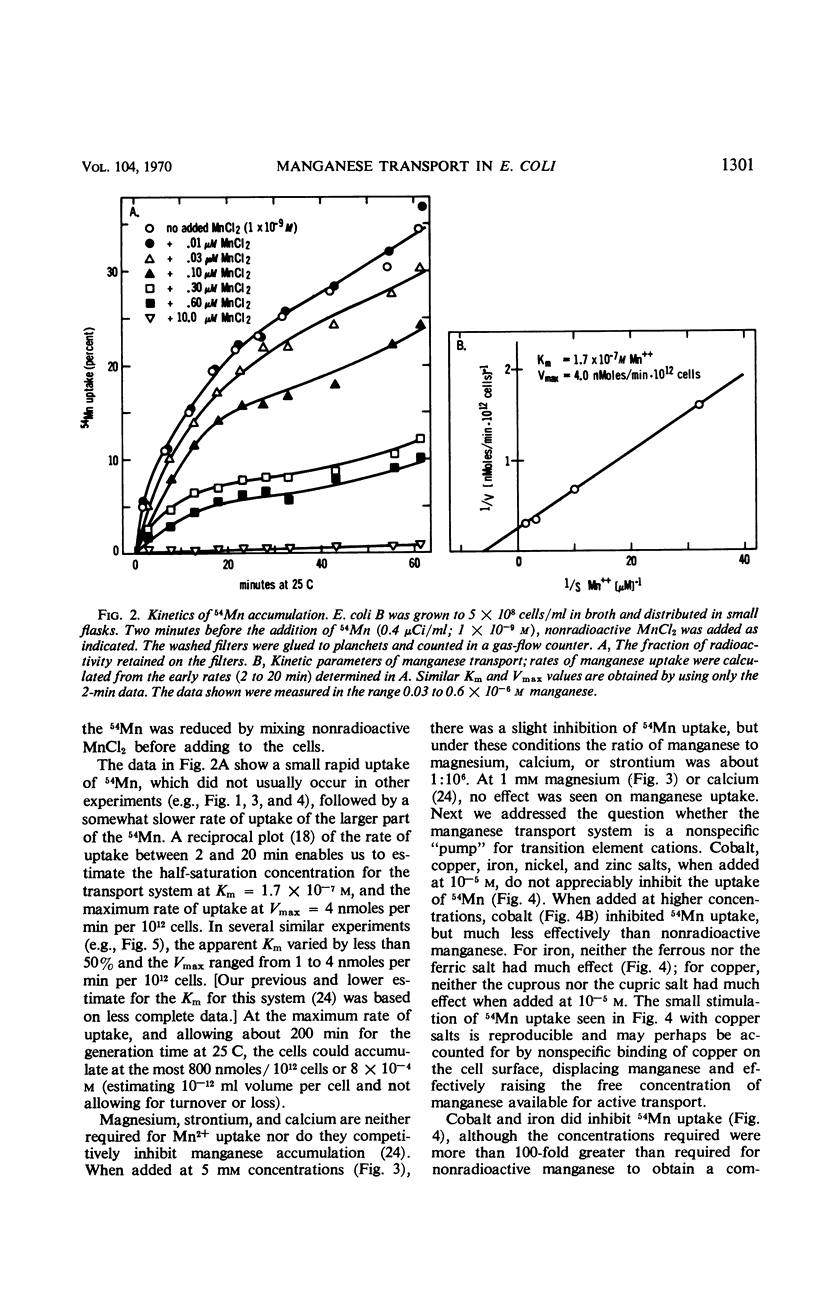
Abstract
Manganese was accumulated by cells of Escherichia coli by means of an active transport system quite independent of the magnesium transport system. When the radioisotope 54Mn was used, manganese transport showed saturation kinetics with a Km of 2 × 10−7m and a Vmax of 1 to 4 nmoles/min per 1012 cells at 25 C. The manganese transport system is highly specific; magnesium and calcium did not stimulate, inhibit, or compete with manganese for cellular uptake. Cobalt and iron specifically interfered with 54Mn uptake, but only when added at concentrations 100 times higher than the Km for manganese. Active transport of manganese is temperature- and energy-dependent: uptake of 54Mn was inhibited by cyanide, dinitrophenol, and m-chlorophenyl carbonylcyanide hydrazone (CCCP). Furthermore, the turnover or exit of manganese from intact cells was inhibited by energy poisons such as dinitrophenol and CCCP.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Meadow P. M., Haskin M. A., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. I. Utilization of uridine diphosphate acetylmuramyl pentapeptide and uridine diphosphate acetylglucosamine for peptidoglycan synthesis by particulate enzymes from Staphylococcus aureus and Micrococcus lysodeikticus. Arch Biochem Biophys. 1966 Sep 26;116(1):487–515. doi: 10.1016/0003-9861(66)90056-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya P. Active Transport of Manganese in Isolated Membranes of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1307–1311. doi: 10.1128/jb.104.3.1307-1311.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Ion metabolism in a potassium accumulation mutant of Escherichia coli B. I. Potassium metabolism. J Bacteriol. 1968 Jan;95(1):113–122. doi: 10.1128/jb.95.1.113-122.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Schultz S. G. Cation transport in Escherichia coli. VI. K exchange. J Gen Physiol. 1966 Jan;49(3):469–481. doi: 10.1085/jgp.49.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURTH J. J., HURWITZ J., ANDERS M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. I. The purification and properties of ribonucleic acid polymerase. J Biol Chem. 1962 Aug;237:2611–2619. [PubMed] [Google Scholar]
- Foradori A. C., Bertinchamps A., Gulibon J. M., Cotzias G. C. The discrimination between magnesium and manganese by serum proteins. J Gen Physiol. 1967 Oct;50(9):2255–2266. doi: 10.1085/jgp.50.9.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann G. F., Rothstein A. The transport of Zn2+, Co2+ and Ni2+ into yeast cells. Biochim Biophys Acta. 1968 Nov 5;163(3):325–330. doi: 10.1016/0005-2736(68)90117-x. [DOI] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Complementation in vivo between structural mutants of alkaline phosphatase from E. coli. J Mol Biol. 1963 Jul;7:13–22. doi: 10.1016/s0022-2836(63)80015-7. [DOI] [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Pavlasova E. Extrusion of sodium and hydrogen ions as the primary process in potassium ion accumulation by Streptococcus faecalis. J Bacteriol. 1970 Jan;101(1):152–159. doi: 10.1128/jb.101.1.152-159.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENNINGS D. H., HOPPER D. C., ROTHSTEIN The participation of phosphate in the formation of a carrier for the transport of Mg++ and Mn++ ions into yeast cells. J Gen Physiol. 1958 May 20;41(5):1019–1026. doi: 10.1085/jgp.41.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. Effects of toluene on Escherichia coli. J Bacteriol. 1965 Nov;90(5):1420–1425. doi: 10.1128/jb.90.5.1420-1425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E. Constancy of uptake during the cell cycle in Escherichia coli. Biophys J. 1968 Dec;8(12):1401–1412. doi: 10.1016/S0006-3495(68)86562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk J. E., Kennedy E. P. Magneisum transport in Escherichia coli. J Biol Chem. 1969 Mar 25;244(6):1653–1655. [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Pogo A. O., Littau V. C., Allfrey V. G., Mirsky A. E. Modification of ribonucleic Acid synthesis in nuclei isolated from normal and regenerating liver: some effects of salt and specific divalent cations. Proc Natl Acad Sci U S A. 1967 Mar;57(3):743–750. doi: 10.1073/pnas.57.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard B., Miall S. H., Peacocke A. R., Walker I. O., Richards R. E. Proton magnetic relaxation studies of the binding of manganese ions to Escherichia coli ribosomes. J Mol Biol. 1967 Sep 28;28(3):389–402. doi: 10.1016/s0022-2836(67)80088-3. [DOI] [PubMed] [Google Scholar]
- Silver S. Active transport of magnesium in escherichia coli. Proc Natl Acad Sci U S A. 1969 Mar;62(3):764–771. doi: 10.1073/pnas.62.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Kralovic M. L. Manganese accumulation by Escherichia coli: evidence for a specific transport system. Biochem Biophys Res Commun. 1969 Mar 10;34(5):640–645. doi: 10.1016/0006-291x(69)90786-4. [DOI] [PubMed] [Google Scholar]
- Silver S., Levine E., Spielman P. M. Cation fluxes and permeability changes accompanying bacteriophage infection of Escherichia coli. J Virol. 1968 Aug;2(8):763–771. doi: 10.1128/jvi.2.8.763-771.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Neuhaus R. C. Reversal of the vancomycin inhibition of peptidoglycan synthesis by cell walls. J Bacteriol. 1968 Aug;96(2):374–382. doi: 10.1128/jb.96.2.374-382.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissieres A., Schlessinger D., Gros F. AMINO ACID INCORPORATION INTO PROTEINS BY ESCHERICHIA COLI RIBOSOMES. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1450–1463. doi: 10.1073/pnas.46.11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Newton A. Iron transport in Escherichia coli: roles of energy-dependent uptake and 2,3-dihydroxybenzoylserine. J Bacteriol. 1969 Jun;98(3):1142–1150. doi: 10.1128/jb.98.3.1142-1150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. The influence of certain trace metals on bacterial growth and magnesium utilization. J Gen Microbiol. 1968 May;51(3):325–335. doi: 10.1099/00221287-51-3-325. [DOI] [PubMed] [Google Scholar]



