Abstract
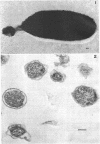
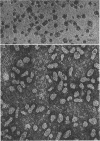
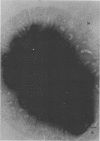
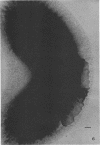
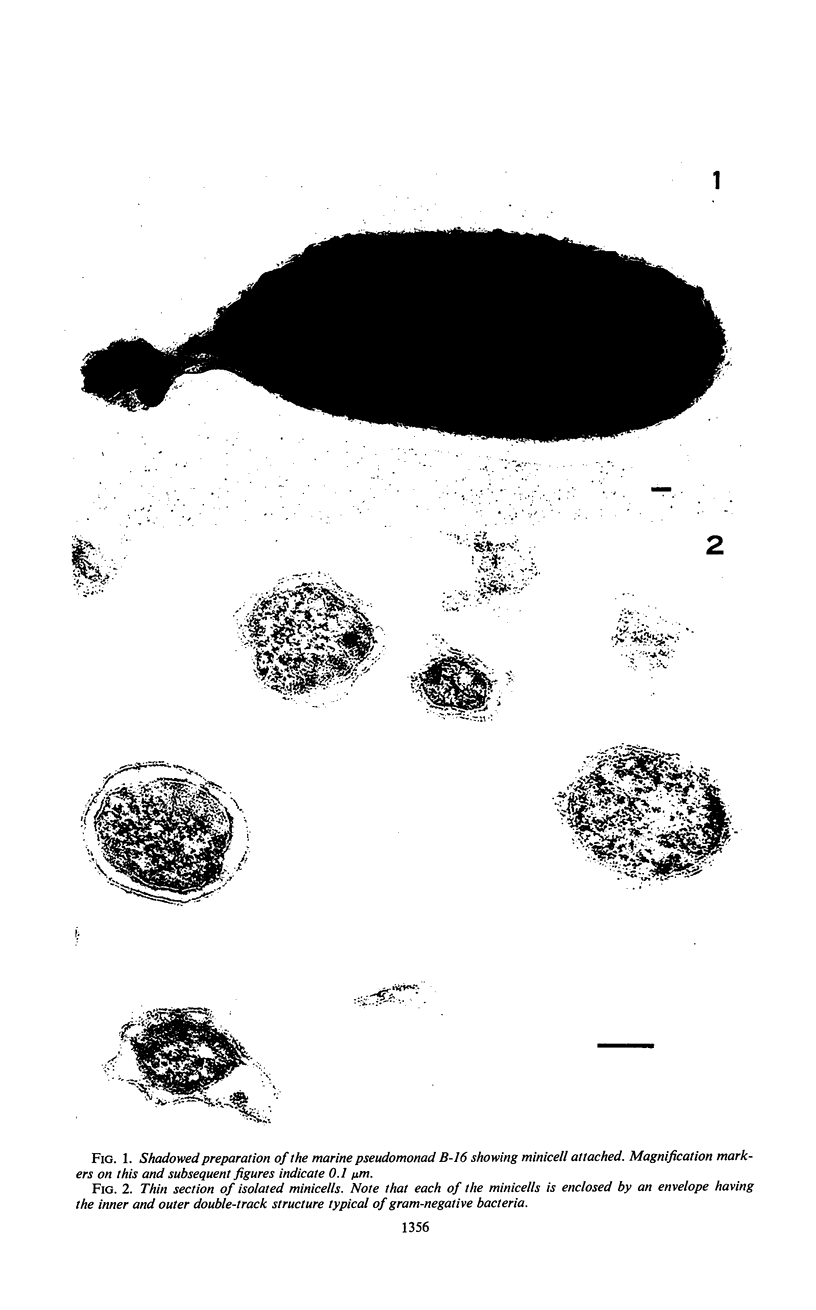
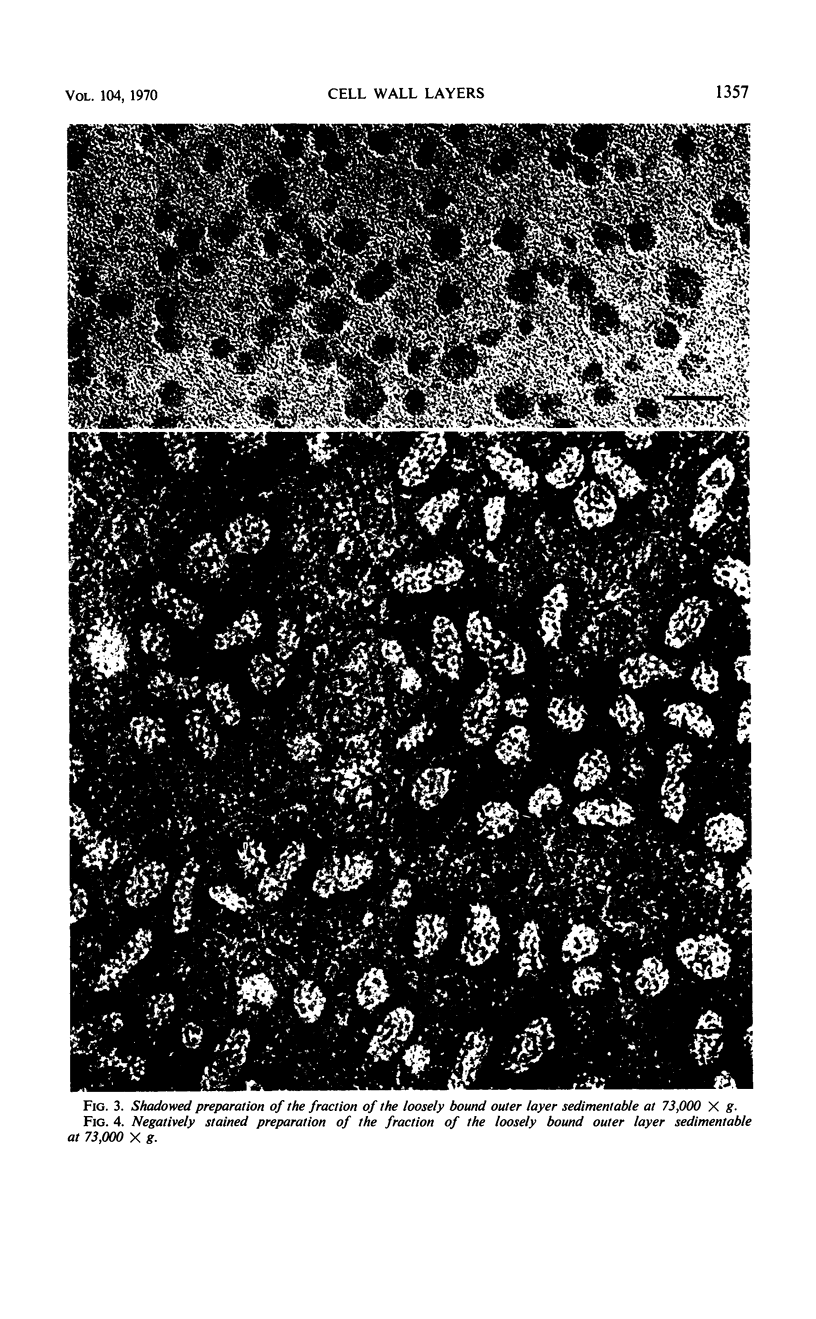
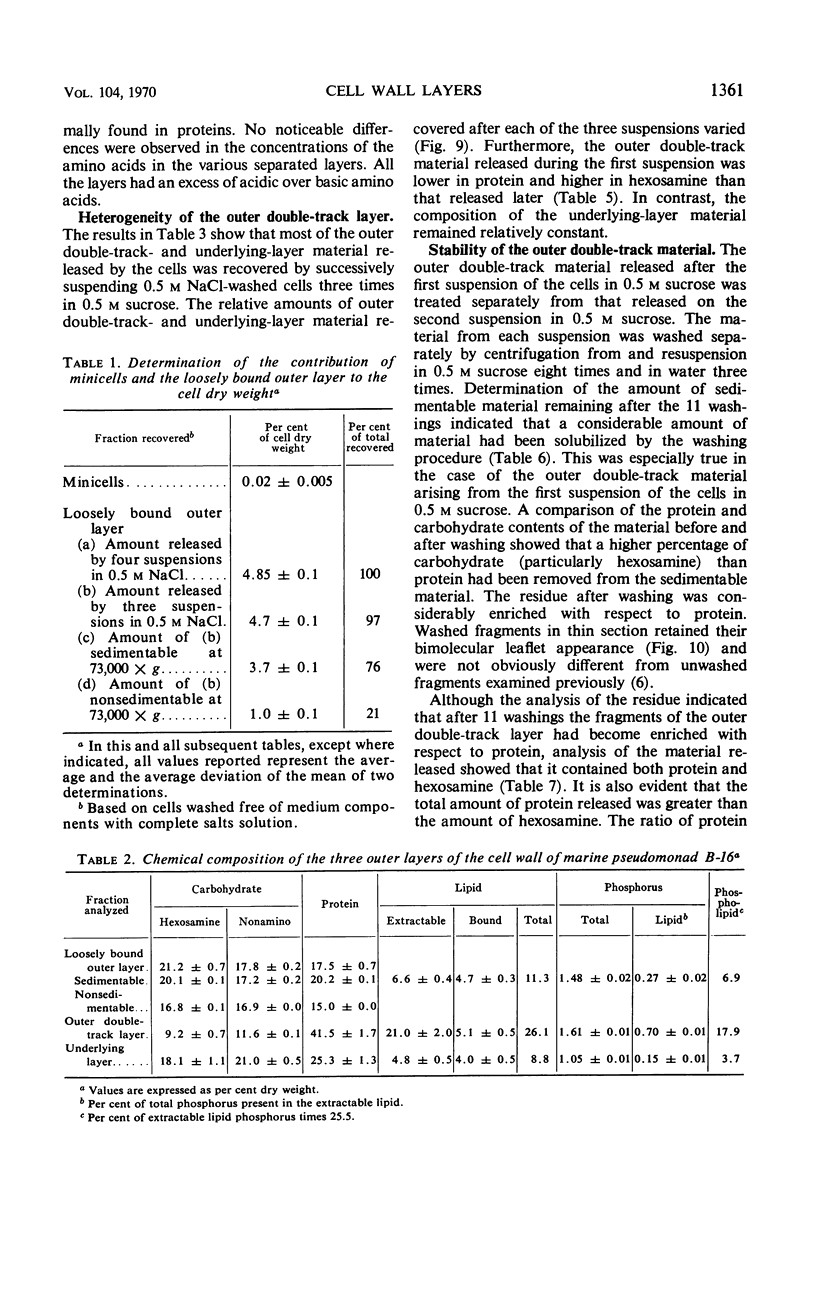
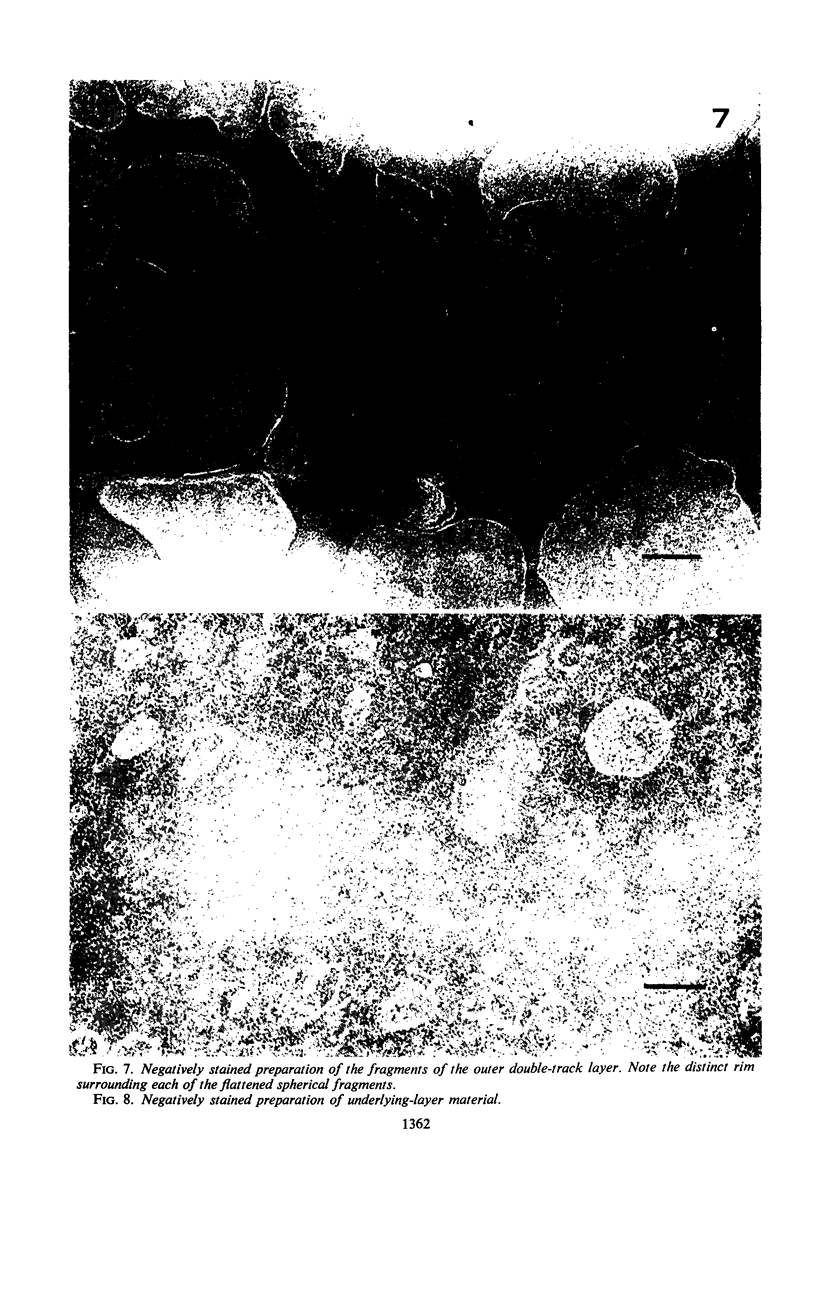
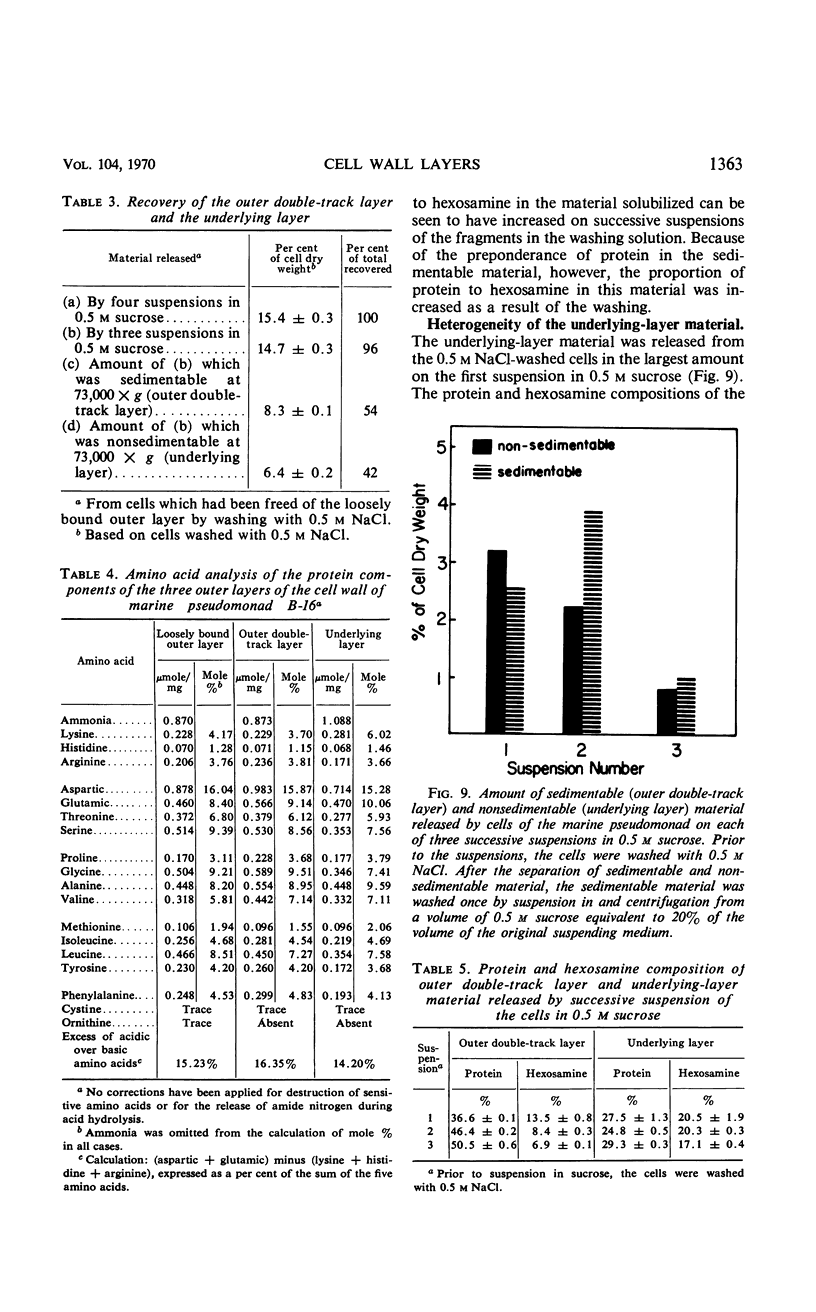
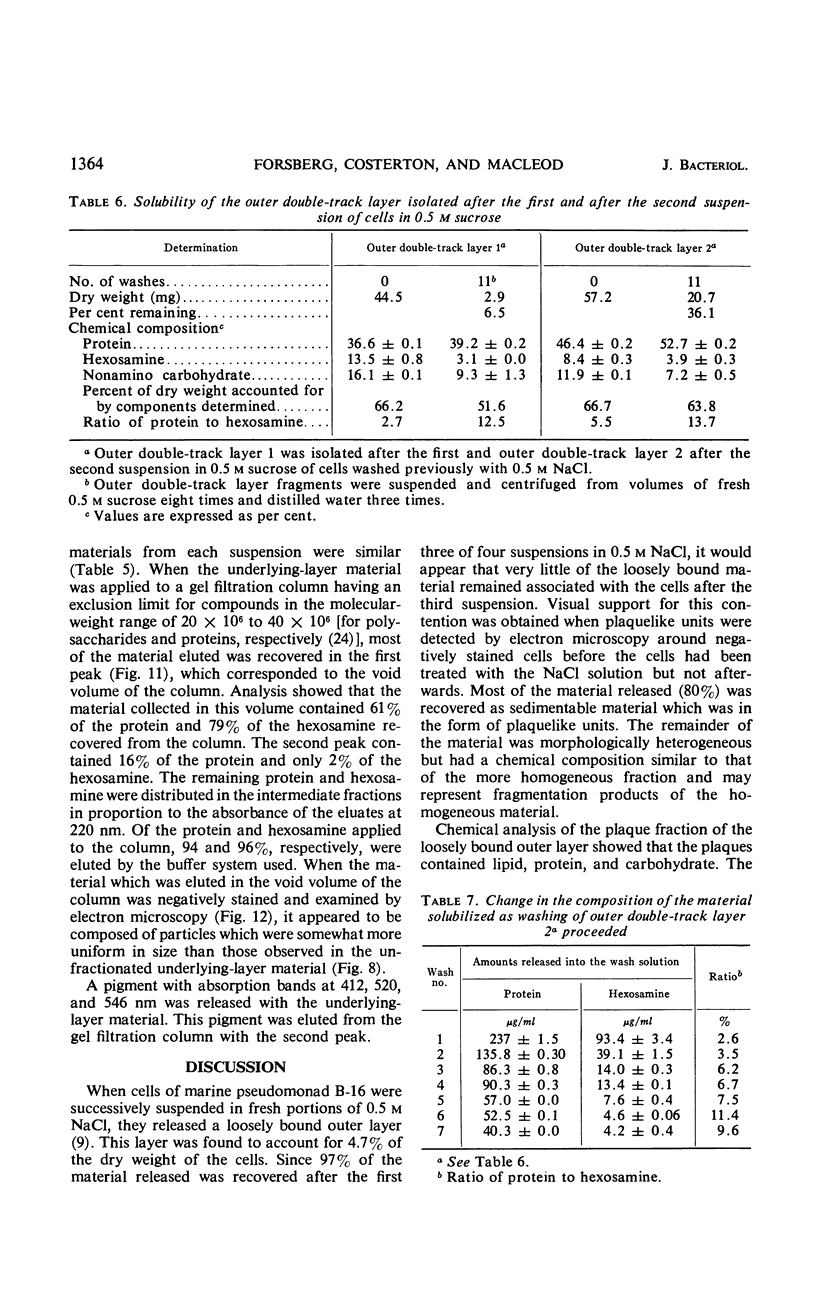
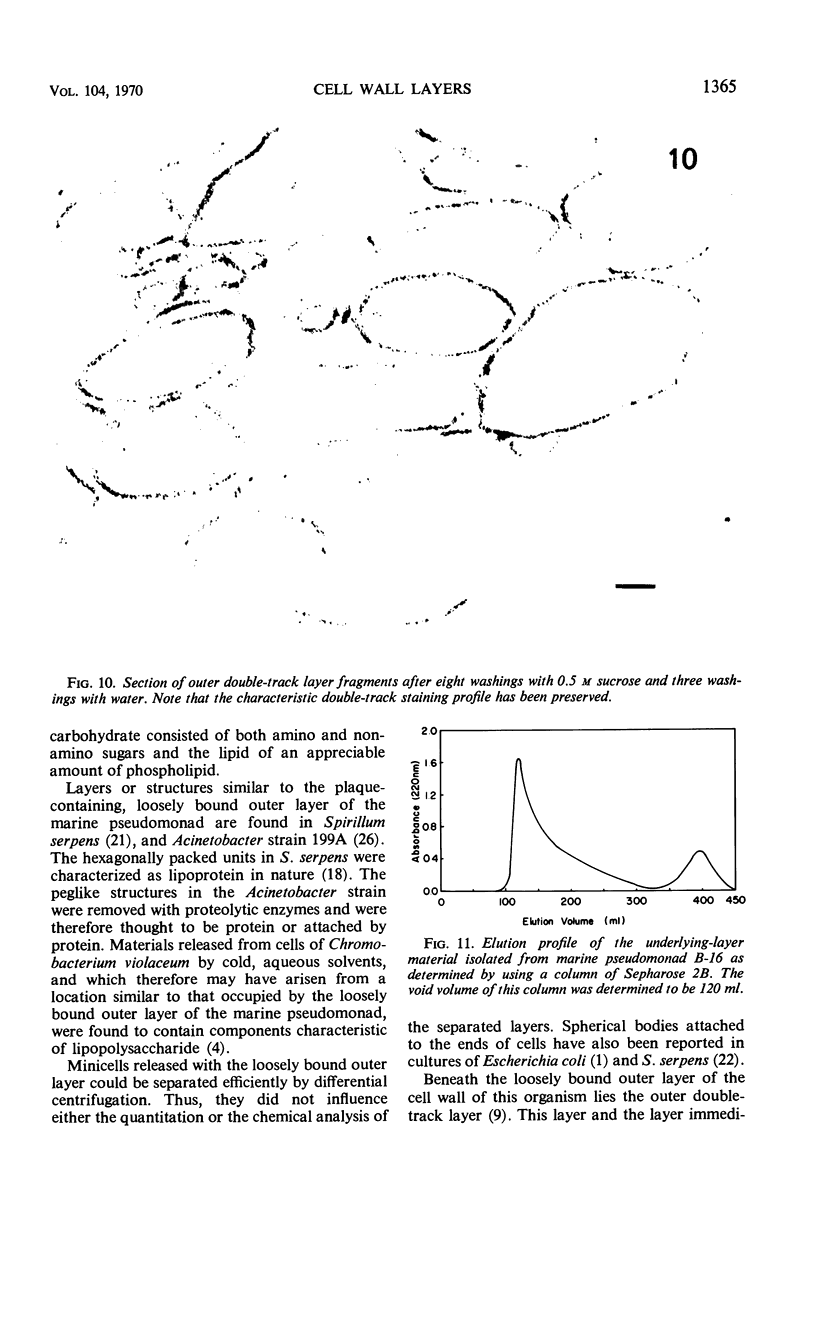
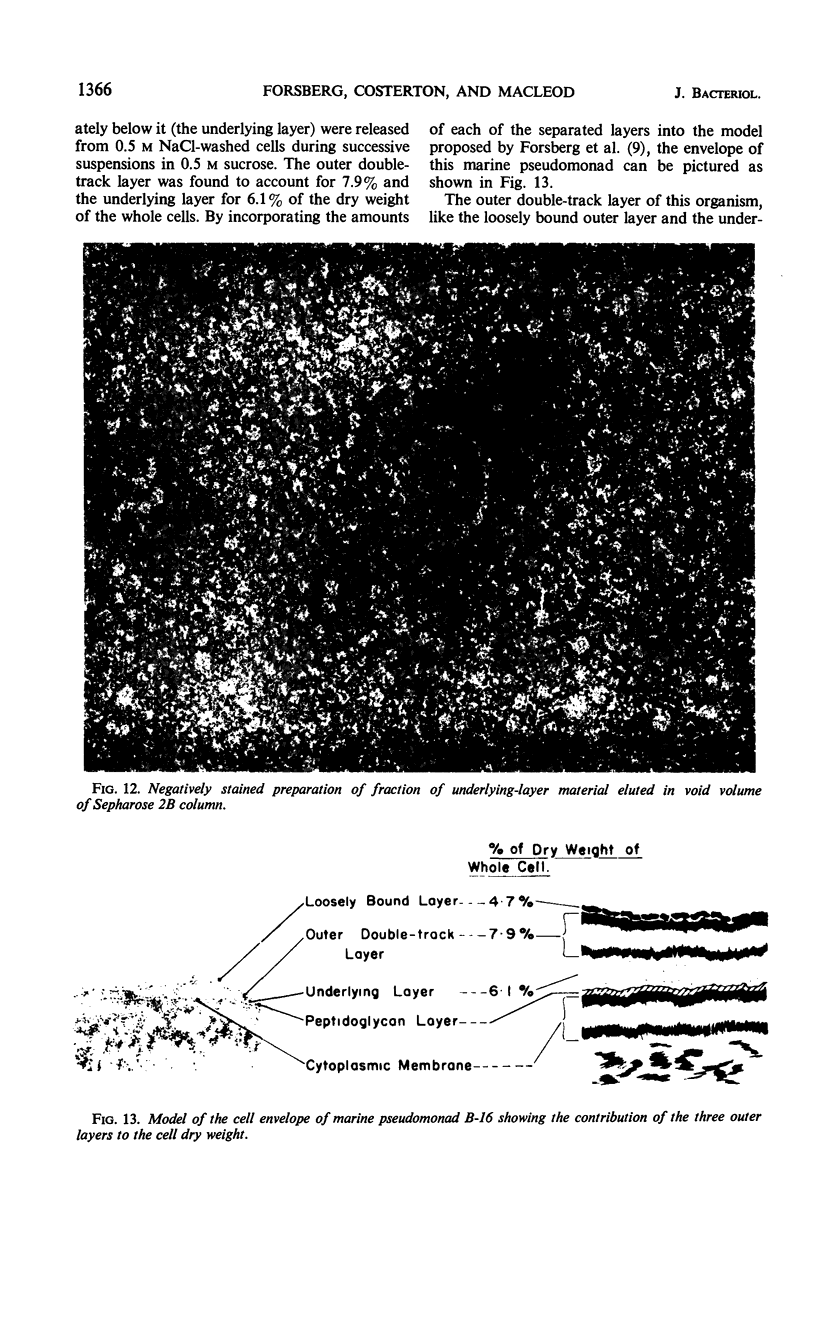
The cell wall of the gram-negative marine pseudomonad (American Type Culture Collection 19855) consists of three layers: the loosely bound outer layer, the outer double-track layer, and the underlying layer. These three layers constitute 4.7, 7.9, and 6.1%, respectively, of the dry weight of the whole cells. All three layers contained protein, lipid, and carbohydrate. The loosely bound outer layer and underlying layer were lower in protein and lipid and higher in amino and nonamino carbohydrate than the outer double-track layer. All three layers contained proteins with similar amino acid compositions. Minicell-like forms attached to the ends of cells were separated with and fractionated from the units of loosely bound outer layer. Examination of negatively stained preparations by electron microscopy revealed the loosely bound outer layer to be composed largely of units ranging from 400 to 1000 nm in diameter. The outer double-track layer, by the same technique, appeared as large, usually rounded sheets, each with a distinct rim. Washing this layer changed the gross chemical composition but did not affect the bimolecular leaflet appearance in thin sections. The underlying layer, when negatively stained, appeared to be composed of a heterogeneous mixture of particles differing in size and shape. It was separated by gel filtration into a large fraction with a molecular weight range in excess of 20 × 106 to 40 × 106 and a small fraction with a lower range of molecular weight. The larger fraction contained both protein and hexosamine, whereas the smaller one contained protein and only traces of hexosamine. A cytochrome-like pigment separated with this latter fraction.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XIV. On the mechanism of lysis of a marine bacterium. Can J Microbiol. 1965 Aug;11(4):677–691. doi: 10.1139/m65-091. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., MacLeod R. A. Penetrability of a marine pseudomonad by inulin, sucrose, and glycerol and its relation to the mechanism of lysis. Can J Microbiol. 1970 Feb;16(2):75–81. doi: 10.1139/m70-014. [DOI] [PubMed] [Google Scholar]
- Corpe W. A., Salton M. R. Properties of surface materials released from cells of Chromobacterium violaceum. Biochim Biophys Acta. 1966 Jul 27;124(1):125–135. doi: 10.1016/0304-4165(66)90320-5. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Forsberg C., Matula T. I., Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVI. Formation of protoplasts, spheroplasts, and related forms from a gram-negative marine bacterium. J Bacteriol. 1967 Nov;94(5):1764–1777. doi: 10.1128/jb.94.5.1764-1777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPETRIS S. ULTRASTRUCTURE OF THE CELL WALL OF ESCHERICHIA COLI. J Ultrastruct Res. 1965 Apr;12:247–262. doi: 10.1016/s0022-5320(65)80098-3. [DOI] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Separation and localization of cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1338–1353. doi: 10.1128/jb.104.3.1338-1353.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN H., FRANK M. E. TIME-LAPSE PHOTOMICROGRAPHY OF THE FORMATION OF A FREE SPHERICAL GRANULE IN AN ESCHERICHIA COLI CELL END. J Bacteriol. 1963 Nov;86:1075–1078. doi: 10.1128/jb.86.5.1075-1078.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSTON C. K., ALBRO P. W. LIPIDS OF SARCINA LUTEA. I. FATTY ACID COMPOSITION OF THE EXTRACTABLE LIPIDS. J Bacteriol. 1964 Aug;88:425–432. doi: 10.1128/jb.88.2.425-432.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hofschneider P. H., Martin H. H. Diversity of surface layers in L-forms of Proteus mirabilis. J Gen Microbiol. 1968 Apr;51(1):23–33. doi: 10.1099/00221287-51-1-23. [DOI] [PubMed] [Google Scholar]
- Homma J. Y., Suzuki N. The protein moiety of the endotoxin of Pseudomonas aeruginosa. Ann N Y Acad Sci. 1966 Jun 30;133(2):508–526. doi: 10.1111/j.1749-6632.1966.tb52386.x. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Cullen J., Work E. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1967 Apr;103(1):192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. H. Bacterial protoplasts--a review. J Theor Biol. 1963 Jul;5(1):1–34. doi: 10.1016/0022-5193(63)90034-1. [DOI] [PubMed] [Google Scholar]
- PEASE P. The gonidial stages in Spirillum spp. and Vibrio spp. J Gen Microbiol. 1956 Jul;14(3):672–675. doi: 10.1099/00221287-14-3-672. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M. Fine structure and radiation resistance in Acinetobacter: studies on a resistant strain. J Cell Sci. 1968 Jun;3(2):273–294. doi: 10.1242/jcs.3.2.273. [DOI] [PubMed] [Google Scholar]