Abstract
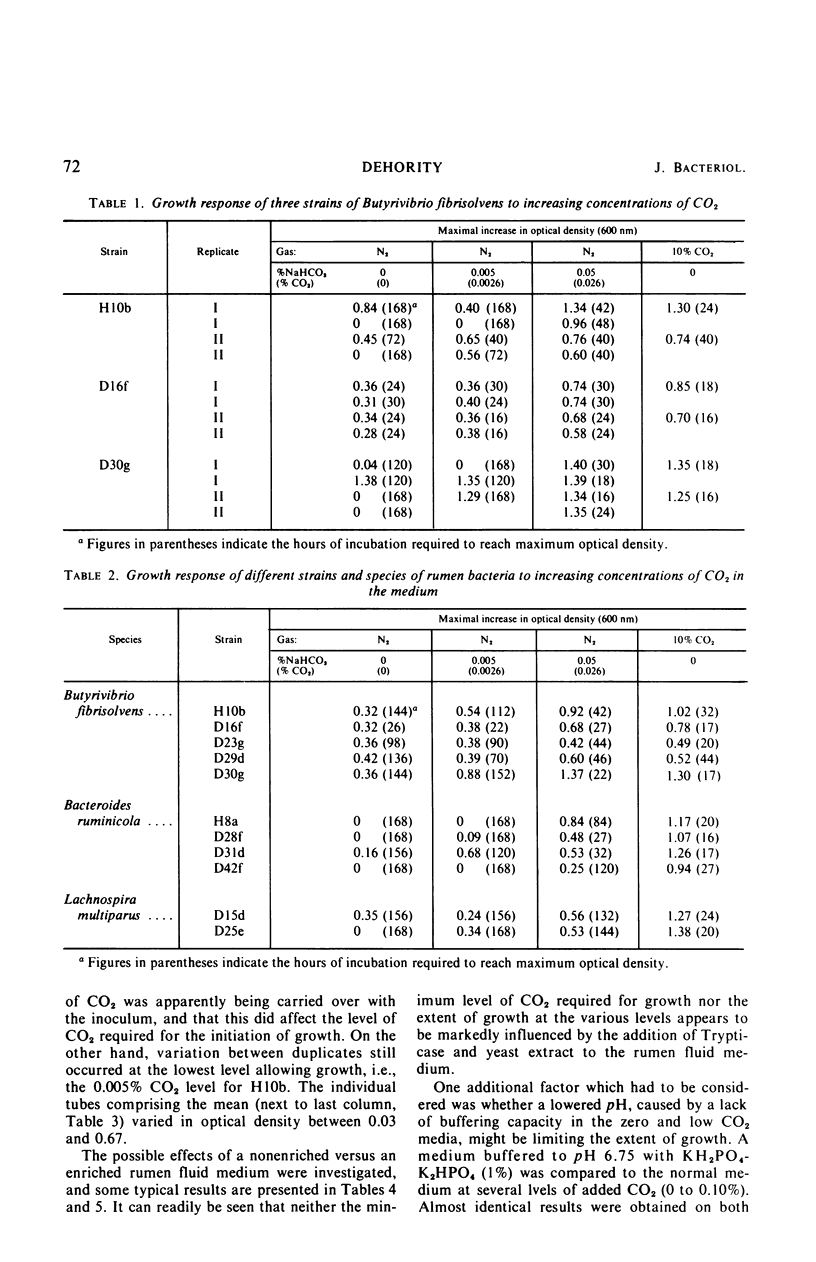
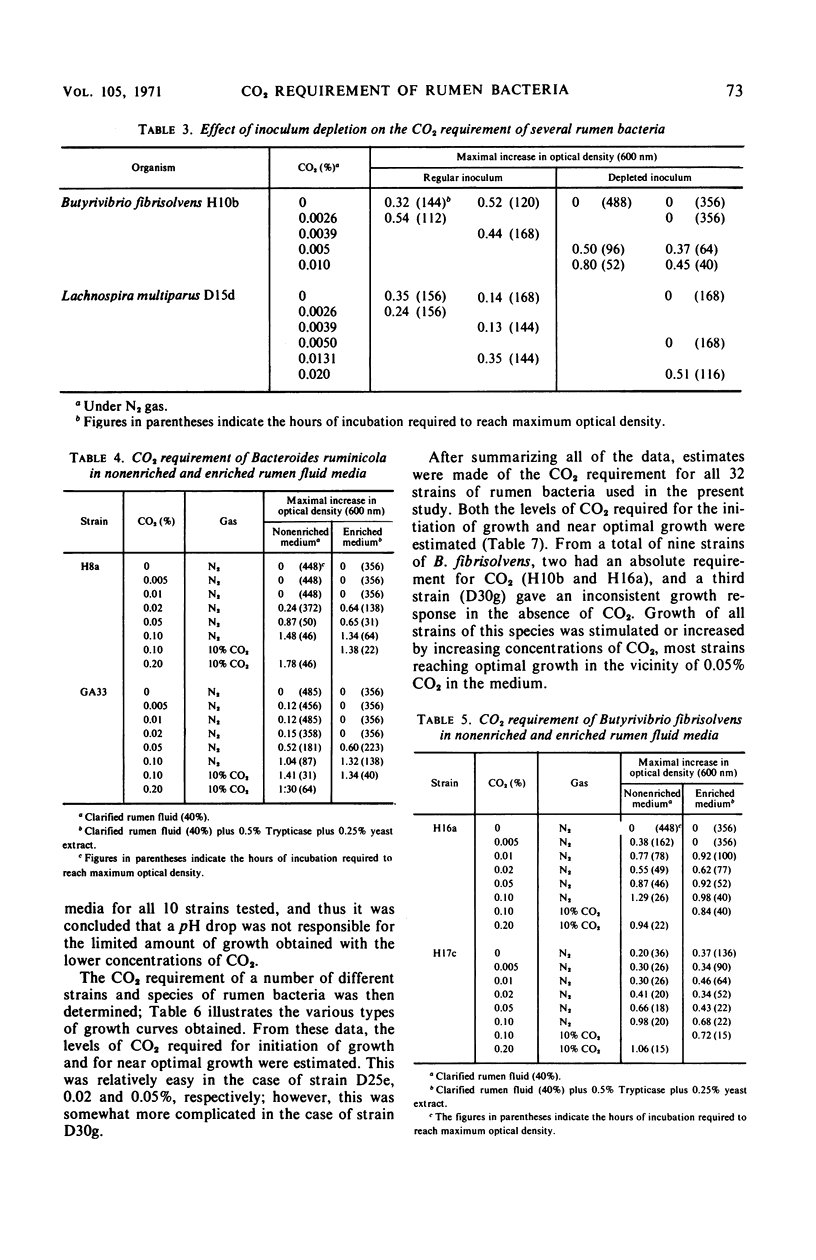
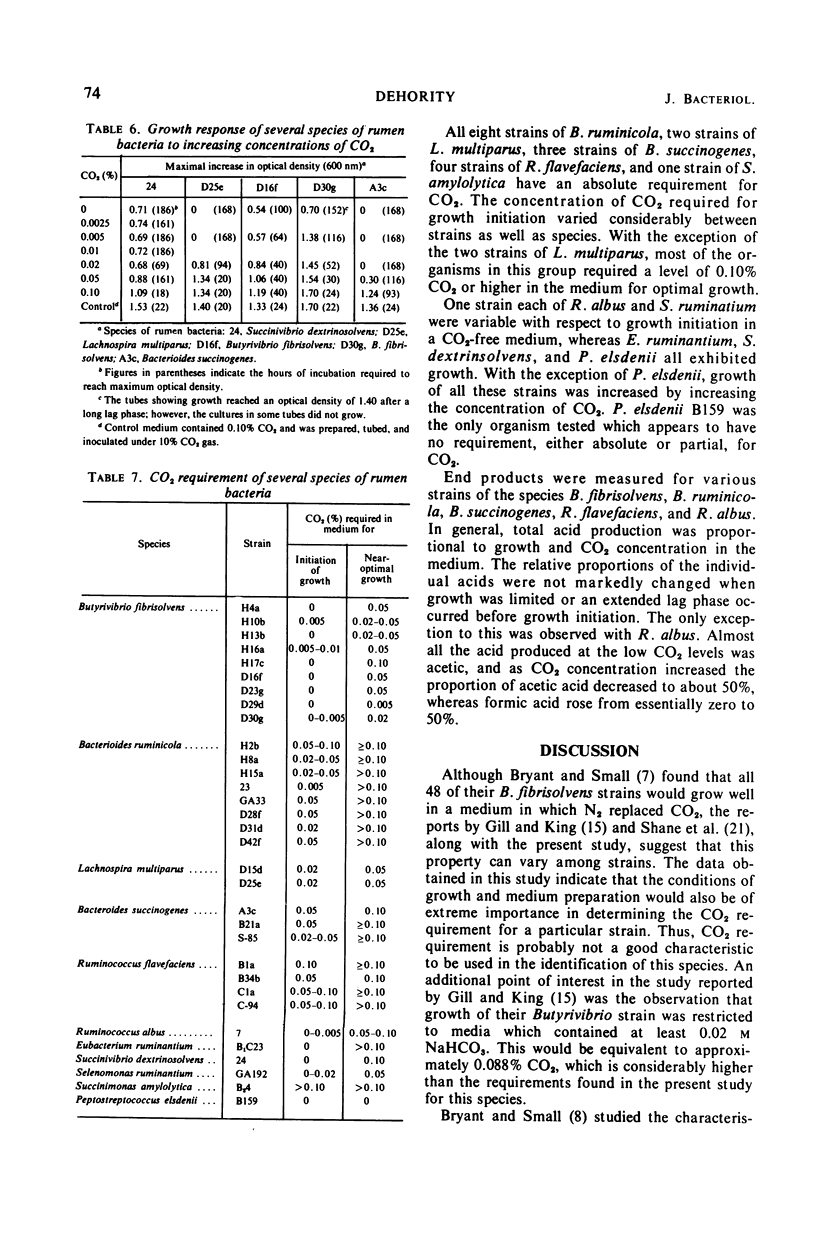
The carbon dioxide requirement of 32 strains of rumen bacteria, representing 11 different species, was studied in detail. Increasing concentrations of CO2 were added as NaHCO3 to a specially prepared CO2-free medium which was tubed and inoculated under nitrogen. Prior depletion of CO2 in the inoculum was found to affect the level of requirement; however, the complexity and buffering capacity of the medium did not appear to be involved. An absolute requirement for CO2 was observed for eight strains of Bacteroides ruminicola, three strains of Bacteroides succinogenes, four strains of Ruminococcus flavefaciens, two strains of Lachnospira multiparus, one strain of Succinimonas amylolytica, and two strains of Butyrivibrio fibrisolvens. Inconsistent growth responses were obtained in CO2-free media with one strain each of B. fibrisolvens, Ruminococcus albus, and Selenomonas ruminantium. Growth of six additional strains of B. fibrisolvens, and single strains of Eubacterium ruminantium and Succinivibrio dextrinosolvens was markedly increased or stimulated by increasing concentrations of CO2. Peptostreptococcus elsdenii B159 was the only organism tested which appeared to have no requirement, either absolute or partial, for CO2. Higher concentrations of CO2 were required for the initiation of growth, as well as for optimal growth, by those species which produce succinic acid as one of their primary end products.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., ROBINSON I. M. Characteristics of ruminal anaerobic celluloytic cocci and Cillobacterium cellulosolvens n. sp. J Bacteriol. 1958 Nov;76(5):529–537. doi: 10.1128/jb.76.5.529-537.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N. Characteristics of two new genera of anaerobic curved rods isolated from the rumen of cattle. J Bacteriol. 1956 Jul;72(1):22–26. doi: 10.1128/jb.72.1.22-26.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N. The anaerobic monotrichous butyric acid-producing curved rod-shaped bacteria of the rumen. J Bacteriol. 1956 Jul;72(1):16–21. doi: 10.1128/jb.72.1.16-21.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P. The characteristics of strains of Selenomonas isolated from bovine rumen contents. J Bacteriol. 1956 Aug;72(2):162–167. doi: 10.1128/jb.72.2.162-167.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Keeney M., Van Soest P. J. Effects of carbon dioxide on growth and maltose fermentation by Bacteroides amylophilus. J Bacteriol. 1969 May;98(2):668–676. doi: 10.1128/jb.98.2.668-676.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Characterization of several bovine rumen bacteria isolated with a xylan medium. J Bacteriol. 1966 May;91(5):1724–1729. doi: 10.1128/jb.91.5.1724-1729.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Pectin-fermenting bacteria isolated from the bovine rumen. J Bacteriol. 1969 Jul;99(1):189–196. doi: 10.1128/jb.99.1.189-196.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUHTANEN C. N., CARLETON F. J., ROBERTS H. R. Carbon dioxide utilization by rumen microorganisms. J Bacteriol. 1954 Dec;68(6):749–751. doi: 10.1128/jb.68.6.749-751.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopgood M. F., Walker D. J. Succinic acid production by rumen bacteria. II. Radioisotope studies on succinate production by Ruminococcus flavefaciens. Aust J Biol Sci. 1967 Feb;20(1):183–192. [PubMed] [Google Scholar]
- PEEL J. L. The breakdown of pyruvate by cell-free extracts of the rumen micro-organism LC. Biochem J. 1960 Mar;74:525–541. doi: 10.1042/bj0740525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESCOTT J. M., RAGLAND R. S., STUTTS A. L. Effects of carbon dioxide on the growth of Streptococcus bovis in the presence of various amino acids. J Bacteriol. 1957 Jan;73(1):133–138. doi: 10.1002/path.1700730116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESCOTT J. M., STUTTS A. L. Effects of carbon dioxide on the growth and amino acid metabolism of Streptococcus bovis. J Bacteriol. 1955 Sep;70(3):285–288. doi: 10.1128/jb.70.3.285-288.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane B. S., Gouws L., Kistner A. Cellulolytic bacteria occurring in the rumen of sheep conditioned to low-protein teff hay. J Gen Microbiol. 1969 Mar;55(3):445–457. doi: 10.1099/00221287-55-3-445. [DOI] [PubMed] [Google Scholar]
- WRIGHT D. E. The metabolism of carbon dioxide by Streptococcus bovis. J Gen Microbiol. 1960 Jun;22:713–725. doi: 10.1099/00221287-22-3-713. [DOI] [PubMed] [Google Scholar]


