Abstract
ERp57 is an oxidoreductase that, in conjunction with calnexin and calreticulin, assists disulfide bond formation in folding glycoproteins. ERp57 also forms a mixed disulfide with the MHC class I-specific chaperone tapasin, and this dimeric conjugate edits the peptide repertoire bound by MHC class I molecules. In cells unable to form the conjugate, because of tapasin mutation in human studies or ERp57 deletion in mouse studies, peptide loading is impeded. Subtle differences between the mouse and human systems have been observed. Here, we address these differences and expand the analysis to investigate the role of ERp57 redox functions in MHC class I peptide loading. We show in human cells that in the absence of conjugate formation MHC class I recruitment and/or stabilization in the MHC class I peptide-loading complex is impaired, similar to observations in mouse cells. However, we found no role for the enzymatic activities of either the a or a′ domain redox sites of ERp57 in peptide loading. Our data argue that the function of ERp57 in peptide loading is likely caused by other ERp57 functional domains or a combinatorial feature of the tapasin–ERp57 conjugate.
Keywords: antigen presentation, antigen processing, human, protein folding, quality control
Stable loading of peptides onto MHC class I/β2-microglobulin (β2m) dimers requires coordinated action within the peptide-loading complex (PLC), which consists of TAP1, TAP2, tapasin, ERp57, calreticulin (CRT), MHC class I heavy chain (HC), and β2m (1). Tapasin is a critical component of the PLC, but the in vivo role of ERp57 in peptide loading and editing is not entirely resolved. ERp57 is an oxidoreductase that promotes proper disulfide bond formation in folding glycoproteins through its association(s) with calnexin (CNX) and/or CRT (2). Like protein disulfide isomerase, ERp57 is composed of four domains with the a and a′ domains containing redox active CXXC motifs. During the biosynthetic folding of MHC class I HC, it appears to act in a manner consistent with models of glycoprotein quality control (3). However, within the PLC, Cys-57 of ERp57 forms a disulfide bond with tapasin Cys-95, and tapasin inactivates the substrate dissociation step, or “escape pathway,” of ERp57, making this interaction very stable (4).
We examined MHC class I assembly in human B lymphoblastoid cells expressing HLA-B*4402 and a tapasin construct in which Cys-95 was mutated to Ala (C95A) to prevent conjugate formation (5). PLC formation was qualitatively normal except for the absence of ERp57, but the stability of peptide–MHC class I complexes assembled in these cells was decreased, consistent with association with a pool of lower-affinity peptides. In mouse B cells lacking ERp57, surface expression of H2-Kb molecules was reduced by ≈50%, and turnover was faster than in ERp57-expressing cells. H2-Kb recruitment into the PLC was affected, and its trafficking through the Golgi was accelerated. Furthermore, presentation of an H2-Kb-restricted epitope derived from ovalbumin was reduced in ERp57-deficient mouse B lymphocytes (6). The tapasin C95A mutation did not affect H2-Kb binding of this ovalbumin-derived peptide, but mutation of Cys-95 prevented the association of H2-Ld with tapasin in human cells (7, 8). Thus, the relative importance of conjugate formation for PLC assembly in mouse and human systems is ambiguous.
A critical question is how ERp57 redox activity is involved in peptide loading. The a domain active site is stably disulfide-linked to tapasin, and this bond would have to be reversibly reduced for this site to have a functional role. The a′ domain site might potentially play a role, and we observed an altered redox state of HLA-B*4402 associated with the PLC in C95A tapasin-expressing cells, which appeared to be consistent with this hypothesis (5). However, MHC class I redox changes were not observed in ERp57-deficient mouse B cells (6). A recent study identified a disulfide-linked complex of MHC class I HC, tapasin, and ERp57 in the PLC, and the authors suggested that the a′ domain cysteines of ERp57 may be required for “triple conjugate” formation (9). However, this hypothesis was not directly demonstrated, and cysteines in the transmembrane or cytoplasmic domains of tapasin and MHC class I HC could mediate these interactions (ref. 10 and D.R.P. unpublished observations). Kienast et al. (11) recently proposed that conjugate formation inhibits ERp57 redox activity, suggesting that redox-active ERp57 might negatively affect peptide loading. These discrepancies are clearly in need of resolution.
Here, we reinvestigate human cells expressing C95A tapasin to reconcile our data with those obtained in the mouse and subsequently examine the role of the two redox domains of ERp57 in peptide loading. Some aspects of peptide loading differ between mice and humans, but conjugate formation is required for the efficient association of MHC class I with the PLC in human cells. After conjugate formation, ERp57 is irreversibly sequestered in the PLC by tapasin, arguing that the ERp57 a domain does not directly function in peptide loading. Additionally, elimination of the redox activities of both the a and a′ domain CXXC motifs does not affect peptide loading onto HLA-B*4402, suggesting that the positive functions of ERp57 in peptide loading are not related to its role as an oxidoreductase.
Results
Impaired PLC Formation in Cells Expressing C95A Tapasin.
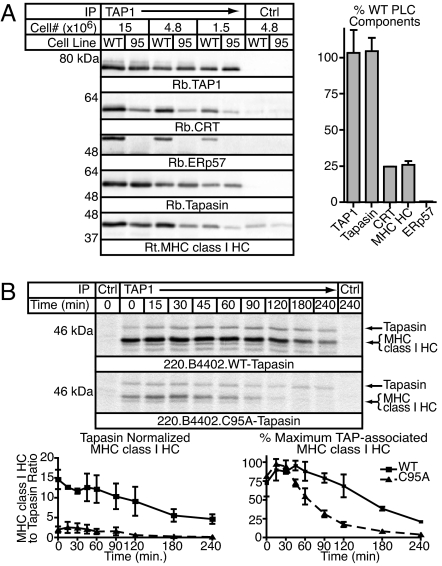
To examine the ability of C95A tapasin to recruit and/or stabilize MHC class I/β2m dimers, known PLC-associated proteins were detected by immunoprecipitation and blotting of extracts of .220 cells expressing HLA-B*4402 and either wild type (WT) or C95A tapasin (Fig. 1A). TAP1 and tapasin levels were identical in cells expressing WT and C95A tapasin, but no ERp57 was associated with C95A-containing PLCs. Both CRT and MHC class I HC were recruited to TAP1 by C95A tapasin, but PLCs from C95A-expressing cells contained only 25% of the levels seen in WT-expressing cells. This level could result from decreased recruitment, accelerated release, and/or loss during detergent solubilization. To determine the cause, we examined the kinetics of MHC class I interaction with the PLC by pulse–chase analysis (Fig. 1B). Throughout the chase, less MHC class I HC was associated with PLCs containing C95A tapasin despite similar tapasin labeling in WT- and C95A-expressing cells. MHC class I associated with WT- and C95A-containing PLCs with similar kinetics, but the duration of the interaction was significantly reduced in the absence of ERp57. Thus, the differences in steady-state MHC class I association with C95A-containing PLCs are at least partially attributable to decreased retention. We cannot distinguish between defects in MHC class I recruitment and postsolubilization loss of MHC class I, but the presence of ERp57 is required for stable formation and maintenance of the complete PLC in human cells, consistent with results obtained in ERp57-deficient mouse B cells and for H2-Ld (6, 7).
Fig. 1.
PLC formation is impaired in C95A-expressing cells. (A) (Left) The steady-state association of MHC class I HC and CRT is reduced in PLCs containing C95A tapasin. WT and C95A-expressing .220.B*4402 cells were treated with MMTS, and digitonin lysates were immunoprecipitated (IP) with 148.3 (TAP1) or B7/21 (Ctrl) coupled to beads. Precipitated material was resolved by reducing SDS/PAGE, and PLC components were detected by immunoblotting with R.RING4C (TAP1), R.CRT (CRT), R.ERp57-C (ERp57), R.gp48N (tapasin), or 3B10.7 (MHC class I HC) and secondary antibodies coupled to alkaline phosphatase. A background band of lower mobility than MHC class I HC consistently present in 3B10.7 blots was excluded from the analysis. All bands were quantitated by fluorometry and were within the linear range of detection. (Right) The relative amount of PLC-associated proteins in C95A-expressing cells was calculated, and the mean ± SEM of three dilutions from two independent experiments is shown. (B) MHC class I PLC association is altered in C95A-expressing cells. Cells from A were pulse-labeled for 15 min and chased for the indicated periods of time before MMTS treatment. Digitonin lysates were immunoprecipitated with R.RING4C (TAP1) or NRS (Ctrl) and protein A–Sepharose, and 0.1% Triton X-100 was used to release tapasin and associated proteins. The eluted material was resolved by reducing SDS/PAGE, and the tapasin and MHC class I HC bands were quantitated. The mean ± SEM of two independent experiments for the MHC class I HC/tapasin ratio (Left) and percentage of maximum TAP-associated MHC class I HC (Right) for WT (■) and C95A (▴) tapasin are shown. In both graphs the curves for WT and C95A tapasin are significantly different, as assessed by two-way ANOVA.
Delayed Maturation of HLA-B*4402 in Cells Expressing C95A Tapasin.
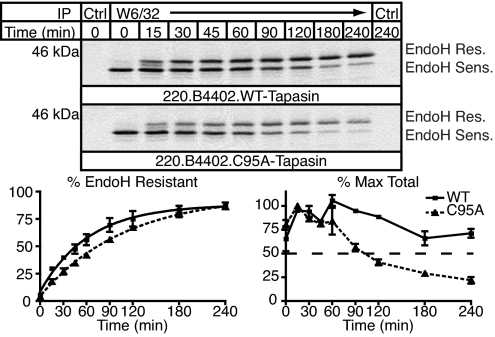
H2-Kb molecules assembled in ERp57-deficient B cells were more rapidly eliminated from the cell surface, a marker of instability, than those assembled in ERp57-expressing cells (6). Similarly, the thermostability and half-life of HLA-B*4402–β2m complexes assembled in C95A-expressing cells were reduced compared with WT-expressing cells (5). In ERp57-negative cells, H2-Kb complexes exited the PLC and traversed the Golgi more rapidly, but our original analysis of C95A-expressing cells did not assess rates of trafficking. Thus, we performed a pulse–chase analysis and harvested cells at 15-min intervals followed by immunoprecipitation with the anti-MHC class I mAb W6/32, endoglycosidase H (EndoH) digestion, and SDS/PAGE (Fig. 2Upper). The acquisition of EndoH resistance by MHC class I/β2m dimers was significantly delayed in C95A-expressing cells (Fig. 2 Lower Left), and fewer W6/32 complexes survived for the 4 h of the experiment (Fig. 2 Lower Right; WT t½ > 4 h, C95A t½ ≈2 h). Those complexes that acquired EndoH resistance were relatively long-lived, however (data not shown), suggesting that passing ER quality control checkpoints may correlate with the loading of higher-quality peptides.
Fig. 2.
MHC class I/β2m dimers mature more slowly and are less stable in C95A-expressing cells. (Upper) Cells were pulse-labeled and solubilized as in Fig. 1B and MHC class I/β2m dimers were immunoprecipitated with W6/32 or 51.1.3 (Ctrl) and protein A–Sepharose. Samples were digested with EndoH before reducing SDS/PAGE, and EndoH-resistant and -sensitive MHC class I HC were quantitated. (Lower Left) The percentage of EndoH-resistant MHC class I HC was calculated, and the best-fit curves for WT (■) and C95A (▴) tapasin are shown. The curves are significantly different (P < 0.0001). (Lower Right) Signals were further expressed as the percentage maximum MHC class I HC, and these curves were significantly different as assessed by two-way ANOVA. The dotted horizontal line corresponds to 50% of the maximum value.
Tapasin-Mediated Inhibition of a Domain Redox Activity Sequesters ERp57 in the PLC.
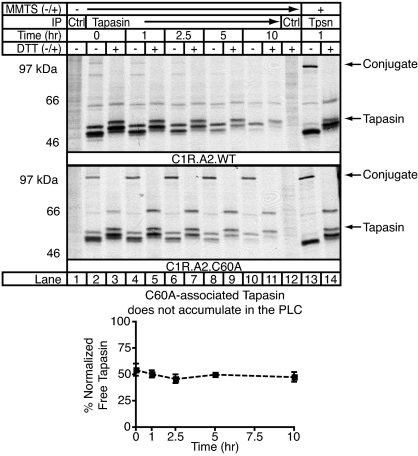
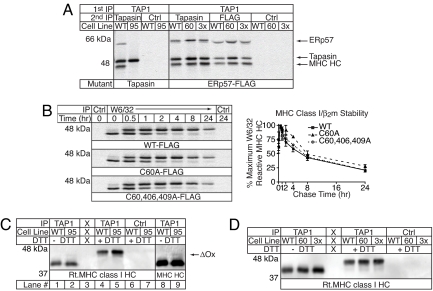
The absence of ERp57 from the PLC affects the association of MHC class I with the PLC and the stability of generated MHC class I/β2m dimers. ERp57 has several functional domains that could mediate these effects. To determine whether the redox active sites of ERp57 are involved, we first asked whether the a domain CXXC motif is active when ERp57 is associated with tapasin. If it is active, exchange should occur between the free and tapasin-conjugated ERp57 pools in the ER. Hence, in cells coexpressing FLAG-tagged C60A ERp57 and endogenous ERp57, the elimination of the a domain escape pathway in the Cys-60 mutant should lead to the accumulation of C60A-FLAG ERp57 in the PLC over time. To test this hypothesis, we labeled and chased WT- or C60A-FLAG ERp57-expressing cells. The cells, not treated with methyl methanethiosulfonate (MMTS) unless indicated, were solubilized in digitonin and immunoprecipitated with PaSta1 before boiling in SDS sample buffer. Eluted material was then resolved by SDS/PAGE under reducing or nonreducing conditions (Fig. 3). As expected, in the cells expressing WT-FLAG ERp57, no conjugate was seen without MMTS treatment after boiling in SDS without reduction (lanes 2, 4, 6, 8, and 10), but in cells where the escape pathway was blocked by MMTS treatment, the conjugate was preserved (lane 13). In contrast, cells expressing both endogenous ERp57 and C60A-FLAG ERp57 had substantial amounts of detectable conjugate in the absence of MMTS treatment (lanes 2, 4, 6, 8, and 10). Conjugates containing C60A-FLAG ERp57 are resistant to escape pathway-mediated conjugate reduction after denaturation in SDS (D.R.P., unpublished observation), and the constant ratio of free to conjugated tapasin argues that C60A-ERp57 does not accumulate in the loading complex with time (Lower). Thus, regulated conjugate reduction through the a domain CXXC motif and ERp57 exchange do not appear to occur during peptide loading. Once incorporated into the MHC class I loading complex, ERp57 is permanently sequestered through inactivation of its a domain redox activity.
Fig. 3.
ERp57 is sequestered in the conjugate. The conjugate does not undergo escape pathway mediated reduction. C1R cells expressing HLA-A*0201 and FLAG-tagged WT or C60A ERp57 were pulse-labeled for 45 min and chased for the indicated times before treatment with or without MMTS as indicated. Digitonin-solubilized cells were immunoprecipitated with PaSta1 (tapasin)- or Ox-68 (Ctrl)-coupled beads, and precipitated material was resolved by SDS/PAGE under reducing or nonreducing conditions. Bands corresponding to tapasin were quantitated, and the amount of free tapasin under nonreducing conditions was normalized to the total amount of tapasin present after reduction. The chart depicts the mean ± SEM of two independent experiments.
Generation of Cells Expressing ERp57 Redox Mutant Conjugates.
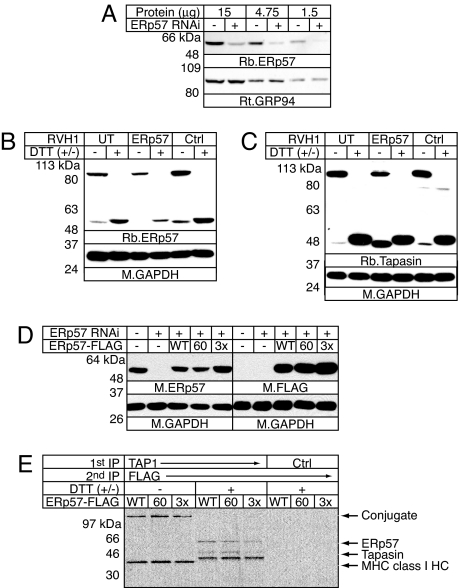
To probe further the function of the a and a′ domain CXXC motifs in peptide loading, we knocked down endogenous ERp57 by using shRNA constructs and then reexpressed FLAG-tagged WT and mutant ERp57. Cells expressing HLA-B*4402 and WT tapasin were transduced with an ERp57-specific shRNA retrovirus, and, after sorting, >90% knockdown was achieved compared with cells transduced with nontargeting shRNA constructs (Fig. 4A). Despite this knockdown, only subtle differences were seen in PLC composition and MHC class I trafficking in these cells (data not shown). These differences are likely because all of the residual ERp57 is conjugated to tapasin, and some tapasin remains in the conjugate (Fig. 4 B and C). These data reinforce our previous finding that ERp57 is recruited preferentially by tapasin in IFN-γ-stimulated cells (4). We next wished to reexpress WT or mutant ERp57 to examine their effects on MHC class I loading. ERp57-suppressed cells were transduced with retroviruses encoding FLAG-tagged WT, C60A mutant, or triple cysteine mutant (C60A/C406A/C409A) ERp57 biscistronically with EGFP and sorted for high EGFP expression. The C60A/C406A/C409A triple redox mutant (3x) combined the trapping feature that inactivates the a domain active site and mutations that inactivate the a′ domain active site. Mutation of Cys-406 and Cys-409 prevents conjugate formation, but the additional mutation of Cys-60 allows conjugation formation to occur (5). As shown in Fig. 4D, ERp57 expression in the transduced cells was comparable to endogenous ERp57. All three ERp57 constructs, including the triple mutant, were incorporated into the PLC and formed the conjugate (Fig. 4E), and virtually all detectable conjugated ERp57 in these cells was FLAG-tagged (Fig. 5A and data not shown). Thus, this system allowed us to specifically address the role of the redox activities of the ERp57 a and a′ domains in MHC class I peptide loading.
Fig. 4.
Successful stable knockdown of ERp57. (A) Generation of ERp57-suppressed cell lines. 721.220 cells expressing HLA-B*4402 and WT tapasin transduced with ERp57-specific or control shRNA sequences were solubilized in 1% Triton X-100. Serial dilutions of ERp57 suppressed (+RNAi) or control cell extracts (−RNAi) were resolved by reducing SDS/PAGE. Proteins were detected by immunoblotting with rabbit anti-ERp57 (R.ERp57-C) and monoclonal rat anti-GRP94 antibody as a loading control. (B) All remaining ERp57 is conjugated to tapasin. Cells from A or untransduced cells (UT) were treated with 10 mM MMTS and solubilized in 1% Triton X-100. Postnuclear supernatants were resolved by reducing/nonreducing SDS/PAGE. Proteins were detected by immunoblotting with rabbit anti-ERp57-C or mouse anti-GAPDH. (C) Conjugate levels are slightly decreased in ERp57-suppressed cells. Cell lysates were prepared as in B, but blots were probed with rabbit anti-tapasin serum (R.SinE) or mouse anti-GAPDH. (D) Expression of FLAG-tagged ERp57. 721.220 cells expressing HLA-B*4402 and WT tapasin with or without ERp57 RNAi and expressing FLAG-tagged WT, C60A, or C60A/C406A/C409A (3x) ERp57 were solubilized in 1% Triton X-100, and postnuclear supernatants were resolved by reducing SDS/PAGE. Total and FLAG-tagged ERp57 were detected by immunoblotting with MaP.ERp57 and M2 (FLAG). Mouse anti-GAPDH was used as a loading control. (E) FLAG-tagged ERp57 is incorporated into the PLC and forms the conjugate. Cells from D were pulse-labeled for 30 min and chased for 30 min. Digitonin lysates of MMTS-treated cells were immunoprecipitated with RING4c (TAP1) or NRS (Ctrl). Triton X-100 (0.1%) was used to release tapasin from TAP, and subcomplexes incorporating FLAG-tagged ERp57 were immunoprecipitated with M2-agarose (FLAG). Samples were resolved by reducing/nonreducing SDS/PAGE as indicated. FLAG-associated PLC components are indicated.
Fig. 5.
Absence of ERp57 redox activity does not affect conjugate function. (A) MHC class I dissociates from unconjugated but not WT or mutant ERp57-conjugated tapasin. Cells as in Figs. 1 and 2 (Left) or Fig. 4E were pulse-labeled as in Fig. 4E. PLCs were precipitated from digitonin lysates of MMTS-treated cells with RING4c (TAP1), and Triton X-100-released material was immunoprecipitated with PaSta1 (tapasin), M2 (FLAG), or Ox-68 (Ctrl) coupled to agarose beads and subjected to SDS/PAGE. Associated PLC components are indicated. (B) MHC class I stability is not affected by the loss of ERp57 redox activity. Cells in A were pulse-labeled for 15 min and chased for the indicated periods of time. MHC class I HC/β2m dimers were immunoprecipitated from digitonin lysates with W6/32 or 51.1.3 (Ctrl) and digested overnight with EndoH. (Left) Samples were resolved by reducing SDS/PAGE and quantitated. (Right) The percentage maximum W6/32-reactive MHC class I HC was calculated, and the mean ± SEM of two independent experiments is shown. (C) A small population of PLC-associated MHC class I HC is partially oxidized in C95A-expressing cells. Cells expressing WT or C95A tapasin from A were treated with MMTS, and PLCs were immunoprecipitated from digitonin lysates by using 148.3 (anti-TAP1) coupled to beads. B7/21 (anti-HLA-DP mAb) coupled to beads was used as a control (Ctrl). Triton X-100-eluted material was resolved by reducing/nonreducing SDS/PAGE, and MHC class I HC was detected by immunoblotting with 3B10.7. Lanes 8 and 9 were overexposed to emphasize the partially oxidized MHC class I HC band (ΔOX) associated with C95A-containing PLCs. (D) PLC-associated MHC class I HC is fully oxidized in the presence of redox-inactive ERp57. Cells expressing WT or mutant ERp57 from A were treated as described in C.
Normal MHC Class I Assembly in the Absence of a and/or a′ Domain Redox Activity.
The three most pronounced phenotypes in cells expressing HLA-B*4402 and C95A tapasin are: impaired incorporation of MHC class I in the PLC, decreased stability of assembled MHC class I complexes, and an altered redox state of MHC class I HC in the PLC (5). Because poor incorporation/stabilization of MHC class I into the PLC likely underlies the defects seen in peptide loading in conjugate-deficient cells, we first examined whether elimination of the redox activity of ERp57 adversely affected PLC stability. Interactions between PLC components are preserved in the detergent digitonin, but incubation of TAP1 immunoprecipitates with Triton X-100 releases subcomplexes containing different combinations of tapasin, ERp57, CRT, MHC class I HC, and/or β2m. Reprecipitation of these eluates from cells expressing WT tapasin with PaSta1 isolates tapasin, ERp57, and associated MHC class I complexes (Fig. 5A Left). When subcomplexes were isolated from Triton X-100 eluates of PLCs from C95A-expressing cells, tapasin is precipitated without ERp57, and MHC class I complexes are lost during biochemical isolation (Fig. 5A Left). Thus, the interaction of MHC class I with unconjugated tapasin is destabilized in Triton X-100, and this correlates with the differences seen at steady-state and in pulse–chase shown in Fig. 1. When the same technique was applied to cells expressing WT, C60A, or C60A/C406A/C409A ERp57, MHC class I remained associated with tapasin after incubation in Triton X-100 (Fig. 5A Right). We further confirmed that MHC class I was associated with the exogenously expressed FLAG-tagged ERp57 by precipitating with an anti-FLAG antibody. Comparable amounts of tapasin, ERp57, and MHC class I HC were precipitated whether WT, C60A, or the triple mutant versions of ERp57 were present (Fig. 5A Right). Thus, elimination of the redox activity of ERp57 does not affect the stabilization of MHC class I with the PLC, and this function of ERp57 is likely attributable to other ERp57 domains or to a combinatorial property of tapasin and ERp57.
Although the interaction of MHC class I with the mutant conjugates is the same as with WT conjugates, there could be effects of the redox domains on the generation of stable MHC class I/peptide complexes. Surface staining of HLA-B*4402 in cells expressing exogenous WT and redox mutant ERp57 was similar (data not shown), but elimination of conjugation by mutation of Cys-95 only decreased HLA-B*4402 surface expression by 50% (5). The most pronounced defects in MHC class I stability in C95A-expressing cells are seen by pulse–chase analysis in both short-term (Fig. 2) and long-term assays (5). However, as seen in Fig. 5B, the long-term (24-h) stability of HLA-B*4402 complexes assembled in cells predominantly expressing redox mutant ERp57 was not significantly different from those assembled in cells expressing WT-FLAG ERp57. Thus, elimination of the a′ and/or a domain CXXC motifs of ERp57 does not adversely affect the generation of stable MHC class I/peptide dimers.
We identified an alteration in the redox state of HLA-B*4402 associated with C95A tapasin, and this finding has recently been expanded (5, 11). There are several possible explanations for these observations, one being that the a′ domain CXXC motif may be required to reoxidize MHC class I HC that becomes reduced during peptide loading. Thus, we wished to examine the redox state of HLA-B*4402 HCs associated with ERp57 mutant-containing PLCs. First, we confirmed that redox changes were apparent in MHC class I HCs in cells expressing C95A tapasin. In contrast to the previous results, only a small fraction of PLC-associated MHC class I HC in C95A-expressing cells was partially reduced (Fig. 5C, lanes 2 and 9). We have no explanation for this difference, but our present results are more similar to those of Kienast et al. (11) than our original observation. However, the absence of the a′ domain active site did not affect the redox status of PLC-associated HLA-B*4402 HCs (Fig. 5D). Taken together, these results indicate that the function(s) of ERp57 within the PLC are largely if not entirely independent of its role as a redox enzyme. Instead, ERp57 likely plays an undefined structural role in recruiting MHC class I complexes into the PLC through its conjugation with tapasin and/or nonredox functions such as interactions with CRT and CNX.
Discussion
Consistent with observations in ERp57-negative mouse B cells, our data argue that tapasin-associated ERp57 is required for the efficient incorporation of MHC class I/β2m dimers into the PLC (6). The use of a sensitive quantitative approach likely explains our ability to demonstrate a decrease in PLC association of MHC class I HC in C95A-expressing cells not revealed in previous studies (5, 11). How conjugate formation leads to MHC class I recruitment and stabilization is unclear. ERp57 has three known functional elements: two redox active sites, in the a and a′ domains, and a CNX/CRT-interacting site in the b and b′ domains (12). However, the a domain redox site is inactivated by tapasin association, and we showed here that there is no exchange of ERp57 molecules conjugated through that site. We further showed that complete inactivation of the thiol exchange capacity of the a domain site by mutation of the C-terminal cysteine residue combined with the elimination of the a′ domain active site does not affect MHC class I incorporation into the PLC. Thus, the redox activity of PLC-associated ERp57 plays no role in MHC class I recruitment. Furthermore, the expression and stability of the MHC class I molecules assembled in the presence of the ERp57 triple mutant are indistinguishable from those assembled in the presence of WT ERp57. Thus, the redox activity also appears to play no role in selecting high-affinity peptides or peptide editing. CNX/CRT binding is the only known functional property of ERp57 that might mediate a direct effect on MHC class I recruitment and peptide loading. Interaction of tapasin-conjugated ERp57 with CRT that is simultaneously bound to the monoglucosylated N-linked glycan of the class I HC may explain why recombinant conjugate can recruit MHC class I/β2m dimers and function as a peptide editor whereas free tapasin cannot (13). Consistent with this, free tapasin acquires the ability to mediate peptide exchange when artificially tethered to MHC class I HC (14).
A substantial fraction of MHC class I-β2m dimers assembled in C95A-expressing cells do not exit the ER, likely because of inefficient recruitment and peptide loading. Those complexes with bound peptide exit the ER and pass through the Golgi, but this population is less stable than the pool that exit the ER in WT cells (5). In contrast, most H2-Kb complexes assembled in ERp57-deficient mouse B cells successfully exit the ER but dissociate in post-ER compartments (6). The reasons for the differences in trafficking between HLA-B*4402 and H2-Kb are unknown, but to exit the ER proteins must pass ER quality control checkpoints. These alleles may differ in their intrinsic ability to pass these checkpoints. Consistent with this possibility, peptide-free H2-Kb molecules expressed in human TAP-deficient cells accumulate at the cell surface whereas TAP-dependent human class I alleles in the same cell do not (15). In addition, surface-expressed H2-Kb molecules in mouse TAP-deficient cells can be stabilized by exogenous β2m or temperature reduction, but neither β2m nor lower temperatures stabilize TAP-dependent human alleles in either mouse or human cells (16).
Kienast et al. (11) recently suggested that the function of the conjugate is to inhibit ERp57 reductase activity toward MHC class I HC. They observed the tapasin-dependent allele HLA-B*4402 in a partially oxidized state in cells lacking tapasin or those expressing C95A tapasin, whereas the tapasin-independent allele HLA-B*4405 was fully oxidized under these conditions. Most of their experiments did not distinguish between pools of MHC class I molecules (e.g., newly synthesized, PLC-associated, ER-retained and targeted for degradation, surface-expressed, etc.) We observed a small population of partially oxidized HLA-B*4402 HCs associated with the PLC in C95A-expressing cells, but much more profound oxidation differences were seen in non-PLC-associated MHC class I HC in the ER (D.R.P., unpublished observations). MHC class I HCs are partially reduced shortly after synthesis and immediately before ER-associated degradation (ERAD), and ERp57 contributes to reduction under both circumstances (3, 17). Mutation of either Cys-101 or Cys-164 in the MHC class I HC peptide-binding groove substantially inhibits peptide loading (11, 18). Conversely, poor peptide loading leads to reduction of that disulfide bond and ERAD. Because C95A tapasin is less efficient at catalyzing peptide loading (5, 19), it is difficult to determine whether nonsequestered ERp57 might inhibit peptide loading by reducing MHC class I molecules or whether reduction occurs because C95A tapasin is inefficient in mediating peptide loading. Generation of tapasin mutants that do not catalyze peptide loading but form the conjugate could resolve this question. Finally, the model is at best incomplete because purified conjugate can promote peptide loading and act as a peptide editor in a cell free assay to a much greater extent than free tapasin (13). This would not be so if the sole purpose of conjugation was to inhibit ERp57 activity toward MHC class I HC.
Soluble conjugate, but not tapasin alone, acts as a peptide editor and exerts a positive function on MHC class I peptide loading (13). Here, we showed that those effects are not the result of a′ domain redox activity. Additionally, the steady-state inhibition of the ERp57 a domain escape pathway we described translates into the permanent sequestration of ERp57 in the conjugate. This strongly suggests that redox regulation by ERp57 does not have a role in MHC class I peptide loading independent of its general role in glycoprotein folding, but the positive function of ERp57 in the PLC remains elusive. H2-Kb trafficking in CRT-deficient fibroblasts is similar to that seen in the absence of ERp57 (20), consistent with the idea that interaction of the bb′ domains of ERp57 with CRT could stabilize MHC class I in the PLC. However, more experiments are needed to evaluate the importance of the ERp57–lectin interactions in MHC class I loading.
Materials and Methods
Plasmids.
Retroviral vectors encoding shRNA constructs targeting ERp57 (RVH1-ERp57) or a control (RVH1-Ctrl) were constructed by using the RVH1 vector (21). The following oligonucleotides synthesized with 5′-phosphates were annealed and ligated as described: ERp57-F, 5′-gat ccc cGG ACT CTT CCA TCA GAG ATt tca aga gaA TCT CTG ATG GAA GAG TCC ttt ttg gaa c-3′; ERp57-R, 5′-tcg agt tcc aaa aaG GAC TCT TCC ATC AGA GAT tct ctt gaa ATC TCT GAT GGA AGA GTC Cgg g-3′; Ctrl-F, 5′-ga tcc ccG CTT CAA CAG CAG GCA CTC ttc aag aga GAG TGC CTG CTG TTG AAG Ctt ttt gga ac-3′; Ctrl-R, 5′-tcg agt tcc aaa aaG CTT CAA CAG CAG GCA CTC tct ctt gaa GAG TGC CTG CTG TTG AAG Cgg g-3′. Correct clones were identified by sequencing. Retroviral vectors encoding WT, C60A, and C60A/C406A/C409A FLAG-tagged ERp57 were generated by ligating fragments from ERp57-FLAG in pCDNA3.1-Puro (5) into the retroviral vector pBMN-IRES-EGFP (a gift of A. Bothwell, Yale University, New Haven, CT).
Cell Lines and Antibodies.
The cell line 721.220 and its transfectants have been described in ref. 5. Cells were first transduced with the RVH1 retroviral shRNA constructs by spinfection and sorted for high CD4 expression with a FACS Vantage SE (22). Cells with stably suppressed ERp57 were then transduced with pBMN-ERp57-IRES-EGFP, and EGFP-positive cells were collected as above. All derivatives of 721.220 were maintained as described in ref. 5. The mAbs used were: 3B10.7 [anti-MHC class I HC (5)], 148.3 [anti-TAP1 (5)], W6/32 [anti-HLA-A, B, C (5)], 51.1.3 [anti-CD1d (23)], BB7.2 [anti-HLA-A2 (24)], PaSta1 [anti-tapasin (5)], M2 (anti-FLAG; Sigma), 6C5 (anti-GAPDH; Research Diagnostics), SPA-850 (anti-GRP94; Stressgen), and B7/21 [anti-HLA-DP (25)]. Rabbit antiserum against TAP1 [R.RING4C (5)], CRT (PA3-900; ABR-Affinity Bioreagents), ERp57 [R.ERp57-C (4)], and tapasin [R.gp48N (26)] were also used. The rabbit antiserum R.SinE was raised against soluble recombinant tapasin.
Pulse–Chase Analyses and Immunoprecipitation.
Pulse–chase analyses were performed as described in ref. 4. For all experiments, starved cells were pulse-labeled with 1 mCi of [35S]Met/Cys labeling mix (ICN or PerkinElmer) per 20 × 106 cells for the indicated times. Harvested, labeled cells were washed once with PBS with or without 10 mM MMTS (Pierce) as indicated and frozen. Cell solubilization in 1% digitonin and preclearing were performed as described in ref. 4. Proteins were immunoprecipitated by incubating with mAbs coupled to Bio-Gel A15m beads or mAbs or antiserum and protein A–Sepharose for 1 h at 4°C followed by washing in 0.1% digitonin. For EndoH digestions, beads were heated to 95°C in 2× EndoH buffer (0.05 M sodium phosphate, 0.25% SDS, pH 6.5) for 5 min. Eluted material was digested with 1 milliunit of EndoH (Roche) overnight at 37°C before reducing SDS/PAGE. TAP1 immunoprecipitates were eluted in 0.1% Triton X-100 (American Bioanalytical) for 5 min on ice, and SDS/PAGE sample buffer was added to eluted material. All other immunoprecipitates were eluted directly into SDS/PAGE sample buffer. Samples were resolved by reducing or nonreducing SDS/PAGE as described in ref. 4. Quantitation was performed with ImageQuant software (GE Healthcare).
Immunoprecipitation and Western Blotting.
Cells were treated with 10 mM MMTS in PBS and extracted as described above. Postnuclear supernatants were precleared with protein A–Sepharose and precipitated with mAb coupled to Bio-Gel A15m beads for 1 h at 4°C. After washing, precipitated material was eluted in 2× reducing SDS/PAGE sample buffer at 95°C for 5 min or with 0.1% Triton X-100 for reducing/nonreducing SDS/PAGE as described above. After transfer, membranes were blocked, probed, and washed, and proteins were detected as described in ref. 4 for quantitative and nonquantitative blots.
Acknowledgments.
We thank Nancy Dometios for aiding in the preparation of this manuscript and our colleague Dr. Pamela Wearsch for reagents and helpful discussions. This work was supported by the Howard Hughes Medical Institute (to P.C.) and the National Institutes of Health/National Institute of General Medical Sciences Medical Scientist Training Grant GM07205 (to D.R.P.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Garbi N, Tanaka S, van den Broek M, Momburg F, Hammerling GJ. Accessory molecules in the assembly of major histocompatibility complex class I/peptide complexes: How essential are they for CD8(+) T cell immune responses? Immunol Rev. 2005;207:77–88. doi: 10.1111/j.0105-2896.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- 2.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Baig E, Williams DB. Functions of ERp57 in the folding and assembly of major histocompatibility complex class I molecules. J Biol Chem. 2006;281:14622–14631. doi: 10.1074/jbc.M512073200. [DOI] [PubMed] [Google Scholar]
- 4.Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 2005;24:3613–3623. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dick TP, Bangia N, Peaper DR, Cresswell P. Disulfide bond isomerization and the assembly of MHC class I–peptide complexes. Immunity. 2002;16:87–98. doi: 10.1016/s1074-7613(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 6.Garbi N, Tanaka S, Momburg F, Hammerling GJ. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol. 2006;7:93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- 7.Turnquist HR, et al. The Ig-like domain of tapasin influences intermolecular interactions. J Immunol. 2004;172:2976–2984. doi: 10.4049/jimmunol.172.5.2976. [DOI] [PubMed] [Google Scholar]
- 8.Howarth M, Williams A, Tolstrup AB, Elliott T. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proc Natl Acad Sci USA. 2004;101:11737–11742. doi: 10.1073/pnas.0306294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos SG, et al. Major histocompatibility complex class I-ERp57–tapasin interactions within the peptide-loading complex. J Biol Chem. 2007;282:17587–17593. doi: 10.1074/jbc.M702212200. [DOI] [PubMed] [Google Scholar]
- 10.Chambers JE, Jessop CE, Bulleid NJ. Formation of a major histocompatibility complex class I tapasin disulfide indicates a change in spatial organization of the peptide-loading complex during assembly. J Biol Chem. 2008;283:1862–1869. doi: 10.1074/jbc.M708196200. [DOI] [PubMed] [Google Scholar]
- 11.Kienast A, Preuss M, Winkler M, Dick TP. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat Immunol. 2007;8:864–872. doi: 10.1038/ni1483. [DOI] [PubMed] [Google Scholar]
- 12.Kozlov G, et al. Crystal structure of the bb′ domains of the protein disulfide isomerase ERp57. Structure. 2006;14:1331–1339. doi: 10.1016/j.str.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol. 2007;8:873–881. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Bouvier M. Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. EMBO J. 2007;26:1681–1690. doi: 10.1038/sj.emboj.7601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 16.Anderson KS, Alexander J, Wei M, Cresswell P. Intracellular transport of class I MHC molecules in antigen-processing mutant cell lines. J Immunol. 1993;151:3407–3419. [PubMed] [Google Scholar]
- 17.Antoniou AN, et al. The oxidoreductase ERp57 efficiently reduces partially folded in preference to fully folded MHC class I molecules. EMBO J. 2002;21:2655–2663. doi: 10.1093/emboj/21.11.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warburton RJ, et al. Mutation of the α2 domain disulfide bridge of the class I molecule HLA-A*0201: Effect on maturation and peptide presentation. Hum Immunol. 1994;39:261–271. doi: 10.1016/0198-8859(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 19.Hughes EA, Hammond C, Cresswell P. Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci USA. 1997;94:1896–1901. doi: 10.1073/pnas.94.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao B, et al. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity. 2002;16:99–109. doi: 10.1016/s1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- 21.Barton GM, Medzhitov R. Retroviral delivery of small interfering RNA into primary cells. Proc Natl Acad Sci USA. 2002;99:14943–14945. doi: 10.1073/pnas.242594499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lackman RL, Cresswell P. Exposure of the promonocytic cell line THP-1 to Escherichia coli induces IFN-γ-inducible lysosomal thiol reductase expression by inflammatory cytokines. J Immunol. 2006;177:4833–4840. doi: 10.4049/jimmunol.177.7.4833. [DOI] [PubMed] [Google Scholar]
- 23.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+CD4−CD8− T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parham P, Brodsky FM. Partial purification and some properties of BB7.2: A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981;3:277–299. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- 25.Royston I, Omary MB, Trowbridge IS. Monoclonal antibodies to a human T cell antigen and Ia-like antigen in the characterization of lymphoid leukemia. Transplant Proc. 1981;13:761–766. [PubMed] [Google Scholar]
- 26.Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8:221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]