Abstract
The prevalence of smoking in schizophrenia patients far exceeds that in the general population. Increased vulnerability to nicotine and other drug addictions in schizophrenia may reflect the impact of developmental limbic abnormalities on cortical-striatal mediation of behavioral changes associated with drug use. Rats with neonatal ventral hippocampal lesions (NVHL), a neurodevelopmental model of schizophrenia, have previously been shown to exhibit altered patterns of behavioral sensitization to both cocaine and ethanol. This study explored nicotine sensitization in NVHLs by testing locomotor activity of NVHL versus SHAM-operated controls over three weeks in response to nicotine (0.5 mg/kg) or saline injections (s.c.) followed by a nicotine challenge delivered to all rats two weeks later. At the beginning of the initial injection series, post-injection locomotor activation was indistinguishable among all treatment groups. However, nicotine but not saline injections produced a progressive sensitization effect that was greater in NVHL rats compared to SHAMs. In the challenge session, rats with previous nicotine history showed enhanced locomotor activation to nicotine when compared to drug naïve rats, with NVHL-nicotine rats showing the greatest degree of activity overall. These results demonstrate that NVHLs exhibit altered short-and long-term sensitization profiles to nicotine, similar to altered long-term sensitization profiles produced by cocaine and ethanol. Collectively, these findings suggest the neurodevelopmental underpinnings of schizophrenia produce enhanced behavioral sensitization to addictive drugs as an involuntary and progressive neurobehavioral process, independent of the acute psychoactive properties uniquely attributed to nicotine, cocaine, or alcohol.
Keywords: dual diagnosis, addiction, schizophrenia, mental illness, nicotine, substance use disorders
1. Introduction
Nicotine dependence is a leading cause of preventable illness and death (CDC, 2006; Mokdad et al., 2004) and is prevalent in schizophrenia patients at 2-to-4 fold greater rates than in the general population (Kuehn, 2006; Regier et al., 1990). Investigating this form of ‘dual diagnosis’ may yield new insights into schizophrenia and nicotine dependence as independent and co-morbid diseases.
Numerous studies have explored whether a relationship exists between nicotine’s unique pharmacological properties, nicotinic acetylcholine receptor (nAChR) functioning in schizophrenia, and the decision to smoke as a volitional act of self-medication. Schizophreniabased nAChR abnormalities could be partially normalized by smoking (Breese et al., 2000; Breese et al., 1997; Durany et al., 2000) and nicotine may improve cognitive deficits of schizophrenia (Depatie et al., 2002; George et al., 2006; Levin and Torry, 1996; Mansvelder et al., 2006)
Since schizophrenia entails increased addictions to many drugs including alcohol, cocaine, and nicotine (Dixon, 1999; Regier et al., 1990), which all provoke mesolimbic dopamine (DA)-mediated behavioral reinforcement (Di Chiara and Imperato, 1988; Volkow, 2004), schizophrenia may also involuntarily accentuate addiction as a general process (Chambers et al., 2001). Prefrontal cortex (PfC) and temporal-limbic systems, including the ventral hippocampus, send glutamatergic projections into, and modulate information processing in the nucleus accumbens (NAc) (Finch, 1996; O'Donnell and Grace, 1995). Schizophrenia-related developmental abnormalities of these regions may enhance short and long-term reactions of the NAc—a key motivational center implicated in addiction—to the DA effects of addictive drugs (Chambers et al., 2001).
In rats, neonatal ventral hippocampal lesions (NVHL) produce a spectrum of schizophrenia-like behavioral and neurobiological features. Peri-adolescent onset of hyper-responsiveness to stress and psychostimulants are reduced with neuroleptics, modeling positive symptoms (Lipska et al., 1993; Lipska and Weinberger, 1994). Deficits in socialization (Becker et al., 1999; Sams-Dodd et al., 1997), working memory (Chambers et al., 1996), and pre-pulse inhibition (Becker et al., 1999; Lipska et al., 1995), encompass negative and cognitive symptoms. Similar to schizophrenic brains, NVHLs produce frontal cortical dendritic atrophy, altered synaptic spine density (Flores et al., 2005), and alterations in GABA and glutamate transmitter systems (Lipska et al., 2003; Stine et al., 2001).
Supporting a neurodevelopmental etiology of addiction vulnerability in schizophrenia, NVHLs accentuate acquisition of cocaine self-administration, post-withdrawal drug-seeking, and drug-induced relapse (Chambers and Self, 2002). Impulsive behavior, a predictor of addiction vulnerability, is accentuated in NVHL rats both before and after cocaine exposure (Chambers et al., 2005). Behavioral sensitization—a process by which involuntary injections of addictive drugs produce long-lasting growth of specific behaviors— is also altered in NVHLs with respect to both cocaine and alcohol (Chambers and Taylor, 2004; Conroy et al., 2007). Because behavioral sensitization is largely dependent on the same neural systems, and DA pharmacological properties of drugs as those implicated in addiction (Kauer and Malenka, 2007; Robinson and Berridge, 1993; Vanderschuren and Kalivas, 2000), these findings suggest NVHLs may produce non-drug specific vulnerabilities to addiction. In the present study, we characterized nicotine sensitization in NVHL rats to determine whether this hypothesis could generalize to nicotine.
2. Materials and Methods
2.1 Subjects and NVHL Surgery
Sprague-Dawley pups were born in house on post-natal day (PD) 0 and maintained in liters under standard housing conditions. On PD 7, male pups weighing 15–18 g were selected for surgery based on Lipska et al. (1993). Under hypothermic anesthesia, pups were taped prone to a stereotaxic platform. A 26S gauge Hamilton needle was inserted through an incision on the dorsal skull into the ventral hippocampal formation (AP −3.0 mm, ML ±3.5 mm, VD −5.0 mm relative to bregma). Pups randomly assigned to lesion group received bilateral infusions of 0.3 µL of 10 µg/µL ibotenic acid in artificial cerebrospinal fluid (aCSF), while the SHAM group received 0.3 µL aCSF. Needles were removed 3 minutes after 135 second infusions to revent backflow. After wound closure with Vetbond (3 M), pups were warmed before returning to their mothers in litters of 4–8 pups, balanced by lesion status. Litters were left undisturbed until weaning (PD 21) when subjects were housed in pairs according to lesion status. Animal care and experiments were conducted according to the Guide for the Care and Use Laboratory Animals, and with approval by Indiana University IACUC.
2.2 Behavioral testing
Beginning on PD 60, motor activity was assessed in 8 plexiglass arenas (43.2 × 43.2 × 30.5 cm) equipped with infrared beam transmitters spanning the x and y axes of the locomotor field (Med Associates, Inc., St. Albans, VT). Beam arrays comprised 16 transmitters crossing 5 cm above the arena floor and spaced 2.7 cm apart. Activity Monitor 5.0 software (Med Associates) quantified beam breaks and transformed this data into locomotor vs. stereotypic range-movement. Beam breaks contributing to horizontal movement of subject’s center of mass were translated into distance traveled, while those occurring without horizontal translation of center of mass were recorded as stereotypic counts. These automated stereotypic counts capture a wide range of non-locomotor movements including those that would be rated as stereotypic gestures by a human rater (e.g. sniffing, grooming), and those that may not be specifically categorized. All test sessions occurred over 12 × 10 minute bins with pre-injection activity measured over the first hour, followed by injection and an hour of post-injection activity (Figure 1). The initial injection series occurred Monday through Friday for three consecutive weeks (15 sessions) during which rats received s.c. injections of saline or nicotine (0.5 mg/kg) according to random drug assignment. Two weeks after completing the injection series, all rats received a single nicotine challenge injection (0.5 mg/kg) in another two hour arena test. Nicotine (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% sterile saline to a dose of 0.5 mg/ ml calculated as the base of the salt and adjusted to a pH 7.4. Injection volume was 1 ml/ kg for both nicotine and saline.
Figure 1.
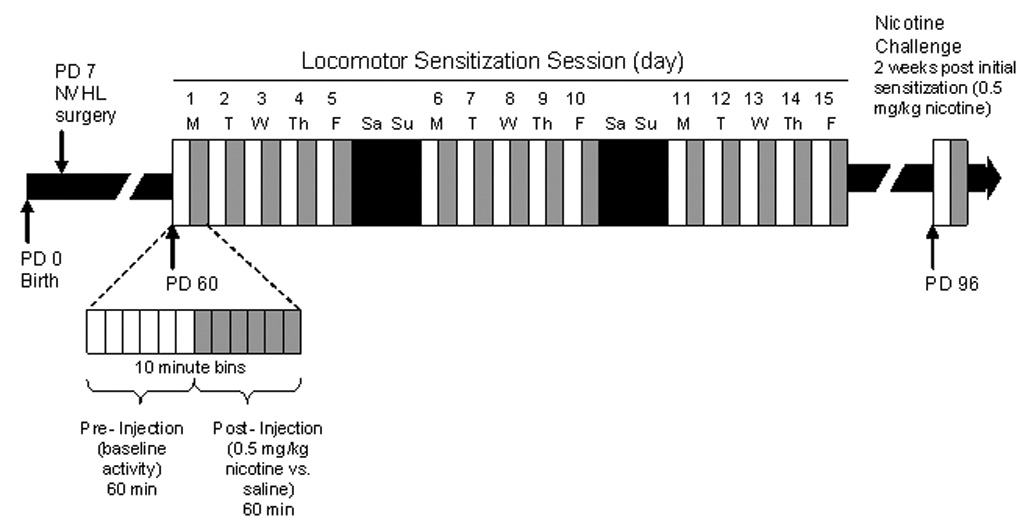
Experimental timeline by rat age (Post-natal Day (PD)). Rats were given either 0.5 mg/kg nicotine or saline during the initial sensitization series. All rats were given 0.5 mg/kg nicotine on the challenge session 2 weeks later.
2.3 Lesion verification
After behavioral testing, subjects were sacrificed under isoflurane anesthesia. Brains were removed and cryostat cut into 40 µm coronal sections every 400 µm from approximately 3.3 to −5.8 mm relative to bregma. Mounted sections were fixed and stained with 0.5% thionin. Appropriate ventral hippocampal damage was assessed microscopically as tissue atrophy, paucity of nuclei, or cellular disarray in at least two serial sections. Rats with unilateral damage, damage extending into the dorsal hippocampus, or beyond the ventral hippocampus into medial or lateral structures were excluded from analysis. Histological analysis confirmed 21 rats with appropriate NVHL and 17 SHAMS. These groups had been randomized by drug group as SHAM-saline (n=8), NVHL-saline (n=10), SHAM-nicotine (n=9) and NVHL-nicotine (n=11). From this qualifying set of animals, lesion extents and micrographs of NVHL and SHAM sections are shown in Figure 2.
Figure 2.
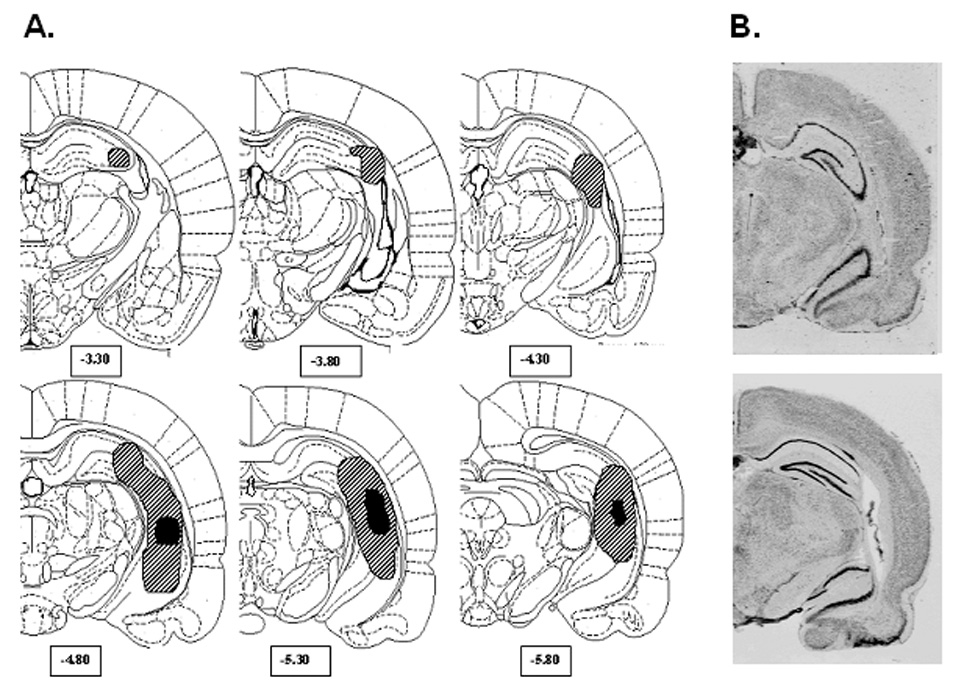
Lesion analysis. (A) Greatest (hatched) and least (solid black) extent of lesion damage allowed for rats included in the study. (B) Coronal sections depicting typical damage observed in a SHAM (top) and neonatal ventral hippocampal lesioned rat (bottom).
2.4 Statistics
Data from day one of the injection series and the final challenge session were examined using two-way Analyses of Variance (ANOVA) testing independent factors of lesion status and drug history on dependent measures of activity. The initial injection series was examined using repeated measures ANOVAs with days as the within subjects measure. For examining whether lesion extent related to NVHL responsiveness to nicotine, the NVHL-nicotine group (n=11) was ordered by visual inspection of lesion extent (total bilaterally), and sub-divided into two groups, those with smaller lesions (n=6) and those with larger lesions (n=5). These two groups were compared in separate secondary analyses over the initial injection series and challenge session. Bonferroni post-hoc analysis was used where indicated. Data is presented as means ±SEM throughout. Significance was taken as p<0.05.
3. Results
3.1 Overview
As shown in Figure 3, locomotor activity within sessions spanning the experiment was generally marked by habituation of activity in the pre-injection hour followed by provocation of a second peak of activation with injections. As suggested by this figure, and analyzed in greater detail below, pre-injection activity curves declined across sessions, while post-injection peaks were progressively elevated in response to nicotine. Through the course of sensitization and in the challenge session, nicotine injections produce a local peak within the first 20 minutes of the post-injection phase that subsided to a plateau that remained higher than with saline injections. The form of these curves did not appear to differ qualitatively between NVHL and SHAM rats.
Figure 3.
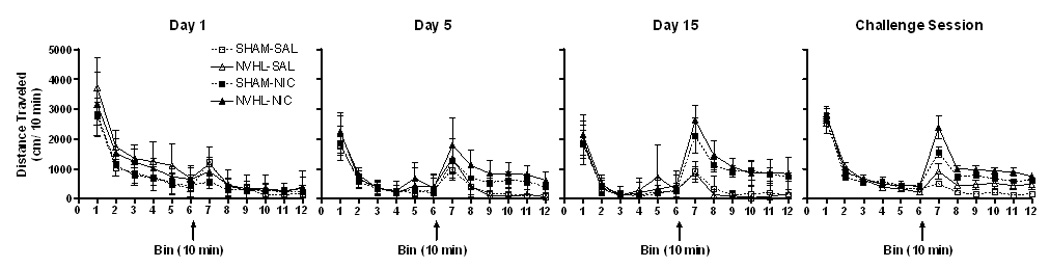
Locomotor activity within sessions. One hour pre-injection activity (bins 1–6) habituated followed by enhanced post-injection activity (bins 7–12). Nicotine and saline injections are denoted by arrows. Data is represented as group means ± SEM.
3.1 Day one
Separate two-way ANOVAs were used to examine pre-injection (novel environment response) and post-injection locomotion. In the pre-injection hour, locomotor activity was greater in NVHLs compared to SHAMs (F(1, 34)=13.9, p<0.01), while drug-group assignment had no effect (Figure 4). No differences were observed in the post-injection hour based on lesion or drug group.
Figure 4.
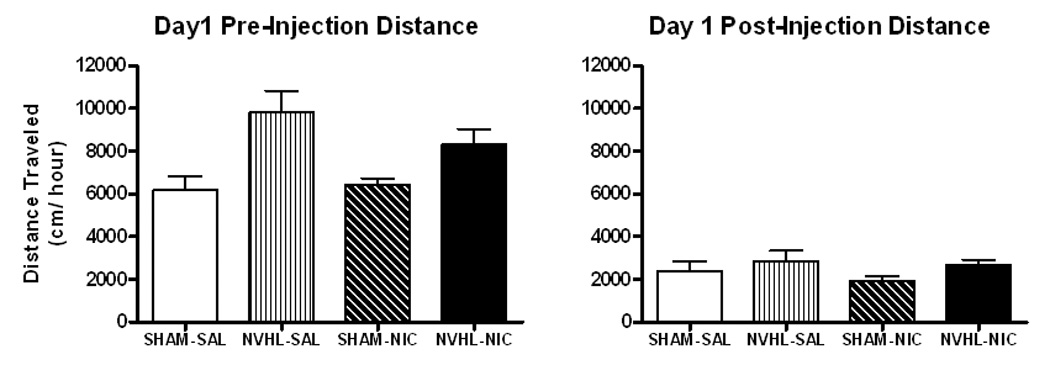
Locomotor activity on day 1. (A) Neonatal ventral hippocampal lesioned rats (NHVLs) had significantly greater pre-injection activity compared to SHAMs. (B) Post-injection activity did not differ between groups by lesion, drug assignment, or their interaction. Data is represented as group means ± SEM.
3.2 Initial Injection Series
Daily pre-injection activity across the 15 day series generally habituated both within and between weeks (Figure 5A). A post-weekend effect observed on days 6 and 11 was marked by minor increases in activation compared to the previous session. In separate repeated measures ANOVAs of pre-injection data for each of the three weeks (Table 1), declining F values for the main effect of days suggested diminishing degrees of habituation on a week to week basis. Lesion-based differences in pre-injection activation and habituation evident in week 1 diminished to non-significance by week three. Drug group assignment had no significant impact or interactions with lesion status in any of the three weeks. To further verify that NVHL-based differences in pre-injection activity could not account for differences in post-injection activity, pre-injection activity was again assessed for the 10-minute bin prior to injection across the entire 15 day injection series with a single repeated measures ANOVA. This testing showed significant habituation across days (F(14,476)=4.038, p<0.001) with no main effects or interactions of lesion or drug group.
Figure 5.
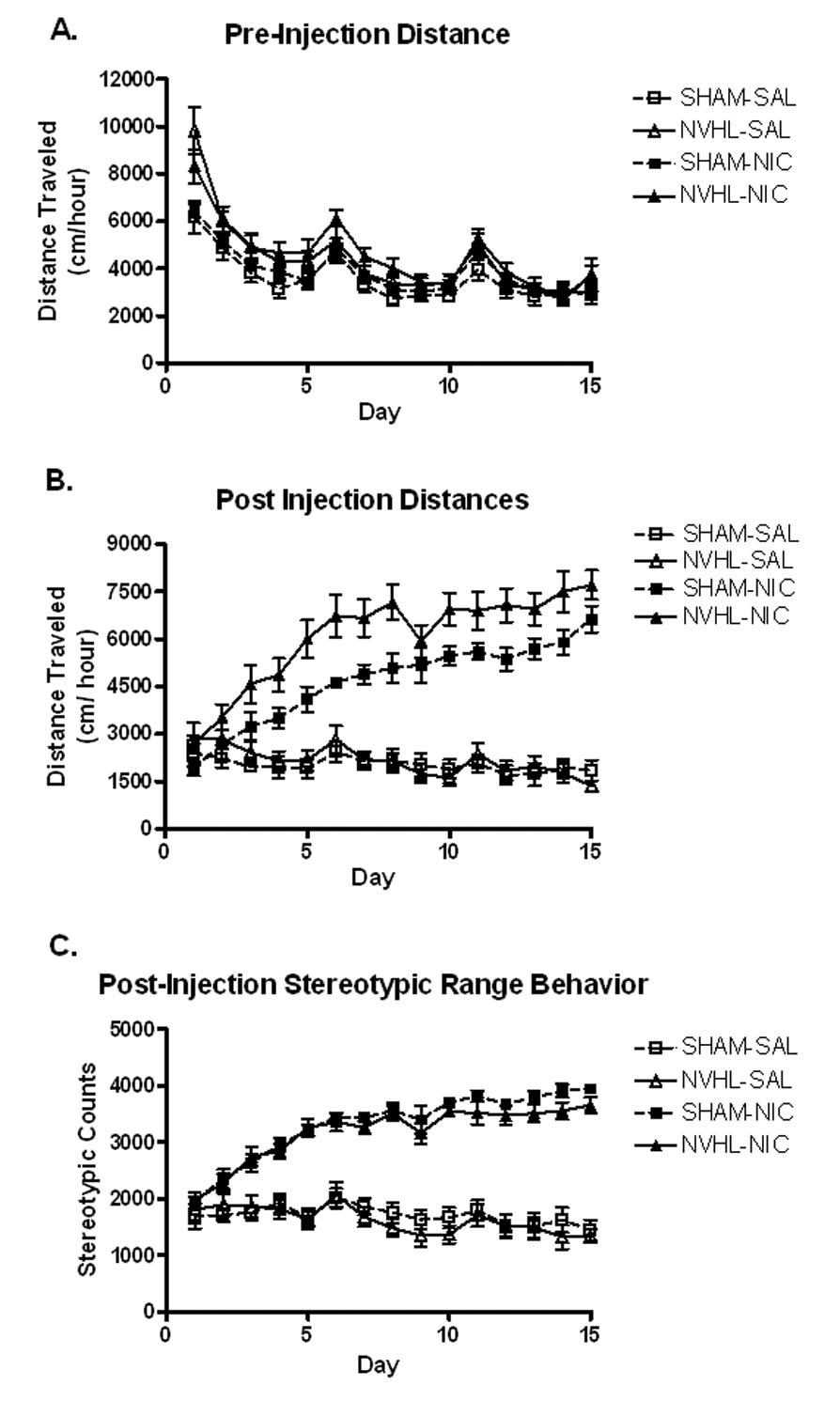
Locomotor activity across the initial injection series. (A) Lesion based differences habituated across the injection series to non-significance in week 3 (Table 1). (B) An overall nicotine sensitization effect was observed in post-injection activity. Neonatal ventral hippocampal lesioned rats (NVHLs) exhibited a greater extent of activation indicated by a significant main effect of lesion and an interaction between drug and lesion status. (C) Nicotine significantly increased post-injection stereotypic range behavior although there was no effect of lesion status. Data is represented as group means ± SEM.
Table 1.
Analyses of weekly pre-injection hour activity. Separate repeated measures ANOVAs with between factors lesion and drug, were performed for each week. Drug assignment had no effects or interactions. Data is depicted graphically in Figure 5A. (N.S: non-significant).
| Week | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Days: F(4, 136), p | 72.3, <0.001 | 33.4, <0.001 | 22.4, 0.001 |
| Lesion: F(1, 34), p | 11.1, <0.01 | 5.8, <0.05 | 1.8, N.S |
| Lesion × Days: F(4, 136), p | 4.8, p<0.01 | 0.7, N.S. | 2.0, N.S. |
Post-injection locomotor activity (Figure 5B) varied across days (F(14, 476)=15.5, p<0.001) with nicotine injections producing overall increases in locomotor activity compared to saline (drug: F(1, 34)=115.2, p<0.001). Nicotine injections also produced progressive increases in activation across the injection series (days × drug: F(14, 476)=29.3, p<0.001) consistent with sensitization in both NVHL and SHAM rats. However, significant lesion (F(1,34)=6.4, p<0.05) and drug × lesion interactions (F(1,34)=4.5, p<0.05) indicated greater overall extents of short-term nicotine sensitization in NVHLs compared to SHAMs. Notably, NVHL rats receiving nicotine reached a mean post-injection activity level by day 5 that was not surpassed by their SHAM counterparts until day 15. Within the NVHL-nicotine group, there were no significant differences in post-injection activation across the 15 day series based on smaller (n=6) or larger (n=5) lesion groupings.
Stereotypic beam-break counts (Figure 5C) also changed across the injection series (days: F(14, 476)=15.175, p<0.001) in which nicotine produced overall increases (drug: F(1, 34)=191.8, p<0.001) and in a sensitizing fashion across days (days × drug: F(4, 476)=28.7, p<0.001). However, there were no main effects or interactions involving lesion status.
3.3 Challenge Session
As in the Day 1 analysis, separate two-way ANOVAs were used to examine pre-and post-injection activity (Figure 6). Here, pre-injection activity did not differ based on lesion, drug history, or their interaction. However, previous nicotine history and NVHLs enhanced post-injection activity as independent factors, and in an additive manner when present in the same group of animals (drug: F(1, 34)=24.0, p<0.001; lesion: F(1, 34)=6.9, p<0.05). Post hoc analyses revealed that activation in saline history NVHL and SHAM rats were not mutually different. However, nicotine history NVHL rats showed greater activity than saline history NVHLs (p<0.01) and saline history SHAMs (p<0.001) while nicotine history SHAMs only showed greater activation then saline history SHAMs (p<0.05). Secondary analyses of NVHL rats with prior nicotine history showed that smaller vs. larger lesion groupings had no significant effect on post-injection activity.
Figure 6.
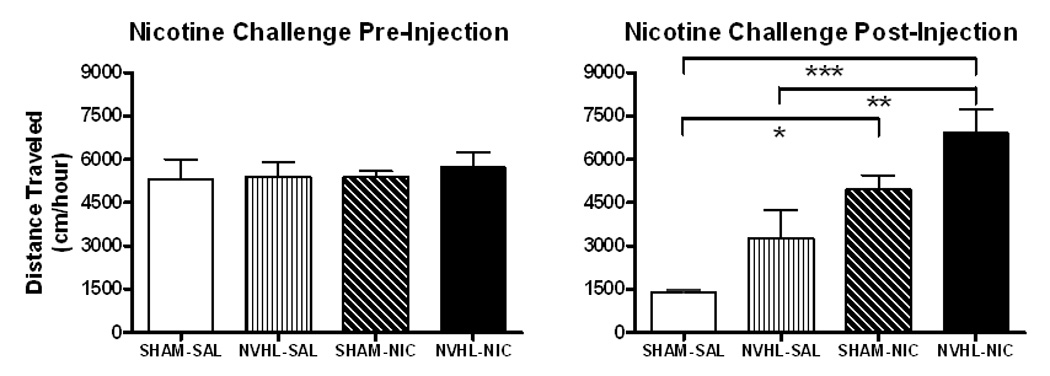
Locomotor activity during the challenge session. (A) No significant differences were observed in pre-injection activity based on lesion, drug history, or their interaction. (B) Post-injection activity was enhanced by previous nicotine history and neonatal ventral hippocampal lesion (NVHL). Bonferoni post hoc analysis revealed that NVHLs have greater long-term nicotine sensitization than do SHAMs. Data is represented as group means ± SEM. *p<0.05; **p<0.01; ***p<0.001.
4. Discussion
Adult NVHL rats show enhanced short and long-term profiles of behavioral sensitization to nicotine. Notably, unlike the psychostimulants cocaine and amphetamine, which show NVHL-based differences on first exposure (Chambers and Taylor, 2004; Lipska et al., 1993), nicotine effects were progressively emergent; a single injection study would not have not detected NVHL differences in nicotine-induced activation. Accentuated progression of nicotine sensitization in the initial injection series was confirmed as an enduring effect in the challenge session, suggesting that prior nicotine exposure has a more profound and/or permanent neuroadaptative impact in this neurodevelopmental model of schizophrenia.
Several aspects of the data indicate these results were not a product of non-specific NVHL hyperactivity. First, post-injection activity in saline-receiving NVHL and SHAM groups was similar across the initial injection series and devoid of a sensitization effect. Second, although replicating previous findings of enhanced NVHL activation to the novel environment (e.g. Lipska et al., 1993), this pre-injection effect normalized to non-significance both across and within sessions, even as the post-injection responses to nicotine became more pronounced. Accordingly, locomotor activity in the 10-minute bin prior to injection across the initial injection series showed no lesion-based differences in locomotor activity. Third, stereotypic-range movement showed no lesion-based differences even though nicotine also sensitized this domain of behavior. Thus, group differences in nicotine-induced locomotion cannot be accounted for by group differences in stereotypic-range behavior which could compete with horizontal locomotion.
The extent to which the present findings simply reflect NVHL-based alterations in nAChR expression/functionality vs. alterations in neural circuits modulated by nAChR activation requires further investigation. However, several lines of evidence suggest the importance of the latter, particularly with respect to mesolimbic DA and prefrontal cortical-striatal circuits that are directly implicated in behavioral sensitization and the addiction process. In contrast to the psychomotoric sensitivity to nicotine characterized here, impaired working-memory performance of NVHL rats is insensitive to modulation by nAChR agonism with nicotine, or antagonism with mecamylamine (Chambers et al., 1996). These findings may reflect loss of hippocampal nAChR fields implicated in the cognitive-supporting roles of acetylcholine and nicotine (Ji et al., 2001; Levin, 2002). In the PfC however, NVHLs increase stress and D1 agonist-mediated acetylcholine release, although it is not known how this might involve changes in nAChR functionality (Laplante et al., 2004a; Laplante et al., 2004b).
NVHLs produce a host of other changes involving the structure and function of both the PfC and NAc, many of which appear to progress through adolescent neurodevelopment in parallel with the behavioral phenotype (Powell et al., 2006; Tseng et al., 2006; Tseng et al., 2007). These changes may collectively account for alterations in the functional impact, and behavioral correlates of endogenous, or nicotine-induced DA release provided by ventral tegmental area (VTA) projections into the PfC-NAc assembly. For instance, while baseline, stress, or amphetamine-induced DA efflux into NAc is not greater in adult NVHL rats compared to SHAMs (Lillrank et al., 1999a; Lillrank et al., 1999b; Wan et al., 1996), NVHL rats show peri-adolescent onset of increased excitability of NAc neurons to VTA stimulation (Goto and O'Donnell, 2002). These NAc responses are not only paralleled by similar abnormalities in PfC responses to VTA stimulation (O'Donnell et al., 2002), but they are abolished by adult lesions to the PfC in NVHL rats, suggesting an interdependency of NVHL-based NAc and PfC functional changes in generating the NVHL phenotype (Goto and O'Donnell, 2004).
In this context of hyper-responsivity to DA stimulation, and to the extent that behavioral sensitization is a DA-dependent process (Everitt and Wolf, 2002; Pierce and Kalivas, 1997; Robinson and Berridge, 1993), enhancement of DA transmission by nicotine and other drugs might be expected to produce comparable abnormalities of long-term behavioral sensitization in NVHL rats. Consistent with this expectation, NVHL rats show elevations in long-term sensitization to both cocaine and alcohol (Chambers and Taylor, 2004; Conroy et al., 2007) analogous to those observed here with nicotine, despite these drugs entailing differential mechanisms of promoting mesolimbic DA efflux (Di Chiara and Imperato, 1988; Wise and Bozarth, 1987) and even more profound differences in a wider array of neurotransmitter and psychoactive effects. Meanwhile, the effects of initial dosing with these drugs in NVHL rats are far less uniform, possibly reflecting a greater contribution of their differential transmitter effects: Cocaine’s robust and relatively selective effects in increasing striatal DA (Ritz et al., 1987) immediately produces hyper-activation in NVHLs (Chambers and Taylor, 2004). Alcohol effects are abnormally stimulating at low doses and normally sedating higher ranges (Chambers and Taylor, 2004; Conroy et al., 2007), consistent with a dose-dependent over-riding of its DA-stimulating effects by its GABA-mediated depressant effects (Dudek et al., 1991; Faingold et al., 1998). In contrast, nicotine was shown here to have no stimulating effects upon initial dosing, yet sensitization progressively emerged. These findings may reflect the unique desensitizing/upregulating dynamics of nicotine on nAChRs (Buisson and Bertrand, 2002; Giniatullin et al., 2005; Nguyen et al., 2004), which may facilitate nAChR stimulation of VTA DA neurons with repeated dosing (Mansvelder et al., 2002; Mansvelder et al., 2006; Wooltorton et al., 2003).
In summary, developmental changes of frontal-cortical striatal circuits generated by NVHLs may represent conditions wherein the cumulative impact of pharmacological DA stimulation has a more potent and/or enduring effect in altering behavioral organization. This study confirms that long-term behavioral sensitization in NVHL rats is involuntarily accentuated with nicotine as with alcohol and cocaine—without necessarily being conditional on the differential mechanisms by which these drugs evoke DA transmission or exert their divergent psychoactive effects. While future studies are needed to understand the neurobiology of the nicotine findings and whether they generalize to other behavioral models of addiction, these results demonstrate utility of the NVHL model in understanding the basis for increased rates of substance disorders transcending addictive drug types in schizophrenia.
Acknowledgments
This work was supported by NIDA K08 DA 019850 (R.A.C.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker A, Grecksch G, Bernstein HG, Hollt V, Bogerts B. Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology (Berl) 1999;144:333–338. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- CDC. C. f. D. C. a. P. Tobacco Use Among Adults- United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1145–1148. [PubMed] [Google Scholar]
- Chambers RA, Jones RM, Brown S, Taylor JR. Natural reward-related learning in rats with neonatal ventral hippocampal lesions and prior cocaine exposure. Psychopharmacology (Berl) 2005;179:470–478. doi: 10.1007/s00213-004-2042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56:308–316. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharmacol Biochem Behav. 2007;86:386–394. doi: 10.1016/j.pbb.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depatie L, O'Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35 Suppl:S93–S100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ, Hahn ME. Genetic analyses of the biphasic nature of the alcohol dose-response curve. Alcohol Clin Exp Res. 1991;15:262–269. doi: 10.1111/j.1530-0277.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Durany N, Zochling R, Boissl KW, Paulus W, Ransmayr G, Tatschner T, Danielczyk W, Jellinger K, Deckert J, Riederer P. Human post-mortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson's syndrome. Neurosci Lett. 2000;287:109–112. doi: 10.1016/s0304-3940(00)01144-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, N'Gouemo P, Riaz A. Ethanol and neurotransmitter interactions--from molecular to integrative effects. Prog Neurobiol. 1998;55:509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus. 1996;6:495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessicchio JC, Duncan EJ. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: involvement of nicotinic receptor mechanisms. Schizophr Res. 2006;87:307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Goto Y, O'Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. J Neurosci. 2002;22:9070–9077. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O'Donnell P. Prefrontal lesion reverses abnormal mesoaccumbens response in an animal model of schizophrenia. Biol Psychiatry. 2004;55:172–176. doi: 10.1016/s0006-3223(03)00783-2. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Link between smoking and mental illness may lead to treatments. Jama. 2006;295:483–484. doi: 10.1001/jama.295.5.483. [DOI] [PubMed] [Google Scholar]
- Laplante F, Srivastava LK, Quirion R. Alterations in dopaminergic modulation of prefrontal cortical acetylcholine release in post-pubertal rats with neonatal ventral hippocampal lesions. J Neurochem. 2004a;89:314–323. doi: 10.1111/j.1471-4159.2004.02351.x. [DOI] [PubMed] [Google Scholar]
- Laplante F, Stevenson CW, Gratton A, Srivastava LK, Quirion R. Effects of neonatal ventral hippocampal lesion in rats on stress-induced acetylcholine release in the prefrontal cortex. J Neurochem. 2004b;91:1473–1482. doi: 10.1111/j.1471-4159.2004.02831.x. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology (Berl) 1996;123:88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Kolachana BS, Weinberger DR. Attenuated extracellular dopamine levels after stress and amphetamine in the nucleus accumbens of rats with neonatal ventral hippocampal damage. J Neural Transm. 1999a;106:183–196. doi: 10.1007/s007020050150. [DOI] [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Weinberger DR, Fredholm BB, Fuxe K, Ferre S. Adenosine and dopamine receptor antagonist binding in the rat ventral and dorsal striatum: lack of changes after a neonatal bilateral lesion of the ventral hippocampus. Neurochem Int. 1999b;34:235–244. doi: 10.1016/s0197-0186(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weickert CS, Weinberger DR. Gene expression in dopamine and GABA systems in an animal model of schizophrenia: effects of antipsychotic drugs. European J Neurosci. 2003;18:391–402. doi: 10.1046/j.1460-9568.2003.02738.x. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. Subchronic treatment with haloperidol and clozapine in rats with neonatal excitotoxic hippocampal damage. Neuropsychopharmacology. 1994;10:199–205. doi: 10.1038/npp.1994.22. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem. 2004;90:40–49. doi: 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Binder TL, Hori S, Nakabeppu Y, Weinberger DR, Lipska BK, Robertson GS. Neonatal ventral hippocampal lesions produce an elevation of DeltaFosB-like protein(s) in the rodent neocortex. Neuropsychopharmacology. 2006;31:700–711. doi: 10.1038/sj.npp.1300883. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. Jama. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Stine CD, Lu W, Wolfe ME. Expression of AMPA receptor flip and flop mRNAs in the nucleus accumbens and prefrontal cortex ater neonatal ventral hippocampal lesions. Neuropsychopharmacology. 2001;24:253–266. doi: 10.1016/S0893-133X(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Amin F, Lewis BL, O'Donnell P. Altered prefrontal cortical metabolic response to mesocortical activation in adult animals with a neonatal ventral hippocampal lesion. Biol Psychiatry. 2006;60:585–590. doi: 10.1016/j.biopsych.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O'Donnell P. Post-Pubertal Disruption of Medial Prefrontal Cortical Dopamine-Glutamate Interactions in a Developmental Animal Model of Schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li T-K. Drug addiction: The neurobiology of behavior gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Wan RQ, Giovanni A, Kafka SH, Corbett R. Neonatal hippocampal lesions induced hyperresponsiveness to amphetamine: behavioral and in vivo microdialysis studies. Behav Brain Res. 1996;78:211–223. doi: 10.1016/0166-4328(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


